Mutations and Modeling of the Chromatin Remodeler CHD8 Define an Emerging Autism Etiology
- PMID: 26733790
- PMCID: PMC4681771
- DOI: 10.3389/fnins.2015.00477
Mutations and Modeling of the Chromatin Remodeler CHD8 Define an Emerging Autism Etiology
Abstract
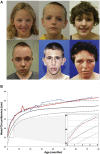
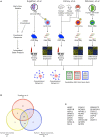
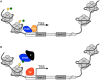
Autism Spectrum Disorder (ASD) is a common neurodevelopmental disorder with a strong but complex genetic component. Recent family based exome-sequencing strategies have identified recurrent de novo mutations at specific genes, providing strong evidence for ASD risk, but also highlighting the extreme genetic heterogeneity of the disorder. However, disruptions in these genes converge on key molecular pathways early in development. In particular, functional enrichment analyses have found that there is a bias toward genes involved in transcriptional regulation, such as chromatin modifiers. Here we review recent genetic, animal model, co-expression network, and functional genomics studies relating to the high confidence ASD risk gene, CHD8. CHD8, a chromatin remodeling factor, may serve as a "master regulator" of a common ASD etiology. Individuals with a CHD8 mutation show an ASD subtype that includes similar physical characteristics, such as macrocephaly and prolonged GI problems including recurrent constipation. Similarly, animal models of CHD8 disruption exhibit enlarged head circumference and reduced gut motility phenotypes. Systems biology approaches suggest CHD8 and other candidate ASD risk genes are enriched during mid-fetal development, which may represent a critical time window in ASD etiology. Transcription and CHD8 binding site profiles from cell and primary tissue models of early development indicate that CHD8 may also positively regulate other candidate ASD risk genes through both direct and indirect means. However, continued study is needed to elucidate the mechanism of regulation as well as identify which CHD8 targets are most relevant to ASD risk. Overall, these initial studies suggest the potential for common ASD etiologies and the development of personalized treatments in the future.
Keywords: CHD8; autism; autism spectrum disorder (ASD); co-expression networks; de novo mutations; functional genomics; subtype; systems biology.
Figures
References
-
- American Psychaitric Association (2013). Neurodevelopmental disorders, in Diagnostic and Statistical Manual of Mental Disorders, 5th Edn (Washington, DC: American Psychiatric Association; ). 10.1176/appi.books.9780890425596.dsm01 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

