NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review
- PMID: 26733801
- PMCID: PMC4683208
- DOI: 10.3389/fnmol.2015.00077
NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review
Abstract
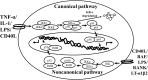
The NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) transcription factor family is a pleiotropic regulator of many cellular signaling pathways, providing a mechanism for the cells in response to a wide variety of stimuli linking to inflammation. The stimulated cells will be regulated by not only the canonical but also non-canonical NF-κB pathways. To initiate both of these pathways, IκB-degradation triggers NF-κB release and the nuclear translocated-heterodimer (or homodimer) can associate with the κB sites of promoter to regulate the gene transcriptions. NF-κB ubiquitously expresses in neurons and the constitutive NF-κB activation is associated with processing of neuronal information. NF-κB can regulate the transcription of genes such as chemokines, cytokines, proinflammatory enzymes, adhesion molecules, proinflammatory transcription factors, and other factors to modulate the neuronal survival. In neuronal insult, NF-κB constitutively active in neuron cell bodies can protect neurons against different injuries and regulate the neuronal inflammatory reactions. Besides neurons, NF-κB transcription factors are abundant in glial cells and cerebral blood vessels and the diverse functions of NF-κB also regulate the inflammatory reaction around the neuronal environment. NF-κB transcription factors are abundant in the brain and exhibit diverse functions. Several central nerve system (CNS) diseases are linked to NF-κB activated by inflammatory mediators. The RelA and c-Rel expression produce opposite effects on neuronal survival. Importantly, c-Rel expression in CNS plays a critical role in anti-apoptosis and reduces the age-related behaviors. Moreover, the different subunits of NF-κB dimer formation can modulate the neuroninflammation, neuronal protection, or neurotoxicity. The diverse functions of NF-κB depend on the subunits of the NF-κB dimer-formation which enable us to develop a therapeutic approach to neuroinflammation based on a new concept of inflammation as a strategic tool in neuronal cells. However, the detail role of NF-κB in neuroinflammation, remains to be clarified. In the present article, we provide an updated review of the current state of our knowledge about relationship between NF-κB and neuroinflammation.
Keywords: NF-kappaB; adhesion molecules; neuroinflammation; neuroprotection; proinflammatory transcription factors.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

