Acid-Sensing Ion Channels Expression, Identity and Role in the Excitability of the Cochlear Afferent Neurons
- PMID: 26733809
- PMCID: PMC4686812
- DOI: 10.3389/fncel.2015.00483
Acid-Sensing Ion Channels Expression, Identity and Role in the Excitability of the Cochlear Afferent Neurons
Abstract
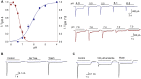
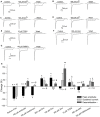
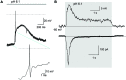
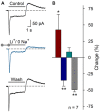
Acid-sensing ion channels (ASICs) are activated by an increase in the extracellular proton concentration. There are four genes (ASIC1-4) that encode six subunits, and they are involved in diverse neuronal functions, such as mechanosensation, learning and memory, nociception, and modulation of retinal function. In this study, we characterize the ASIC currents of spiral ganglion neurons (SGNs). These ASIC currents are primarily carried by Na(+), exhibit fast activation and desensitization, display a pH50 of 6.2 and are blocked by amiloride, indicating that these are ASIC currents. The ASIC currents were further characterized using several pharmacological tools. Gadolinium and acetylsalicylic acid reduced these currents, and FMRFamide, zinc (at high concentrations) and N,N,N',N'-tetrakis-(2-piridilmetil)-ethylenediamine increased them, indicating that functional ASICs are composed of the subunits ASIC1, ASIC2, and ASIC3. Neomycin and streptomycin reduced the desensitization rate of the ASIC current in SGNs, indicating that ASICs may contribute to the ototoxic action of aminoglycosides. RT-PCR of the spiral ganglion revealed significant expression of all ASIC subunits. By immunohistochemistry the expression of the ASIC1a, ASIC2a, ASIC2b, and ASIC3 subunits was detected in SGNs. Although only a few SGNs exhibited action potential firing in response to an acidic stimulus, protons in the extracellular solution modulated SGN activity during sinusoidal stimulation. Our results show that protons modulate the excitability of SGNs via ASICs.
Keywords: ASIC; Corti; FMRFamide; acetylsalicylic acid; aminglycosides; auditory; inner ear; spiral ganglion.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources

