Ion Channels in Native Chloroplast Membranes: Challenges and Potential for Direct Patch-Clamp Studies
- PMID: 26733887
- PMCID: PMC4686732
- DOI: 10.3389/fphys.2015.00396
Ion Channels in Native Chloroplast Membranes: Challenges and Potential for Direct Patch-Clamp Studies
Abstract
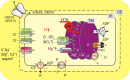
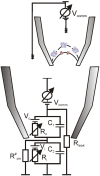
Photosynthesis without any doubt depends on the activity of the chloroplast ion channels. The thylakoid ion channels participate in the fine partitioning of the light-generated proton-motive force (p.m.f.). By regulating, therefore, luminal pH, they affect the linear electron flow and non-photochemical quenching. Stromal ion homeostasis and signaling, on the other hand, depend on the activity of both thylakoid and envelope ion channels. Experimentally, intact chloroplasts and swollen thylakoids were proven to be suitable for direct measurements of the ion channels activity via conventional patch-clamp technique; yet, such studies became infrequent, although their potential is far from being exhausted. In this paper we wish to summarize existing challenges for direct patch-clamping of native chloroplast membranes as well as present available results on the activity of thylakoid Cl(-) (ClC?) and divalent cation-permeable channels, along with their tentative roles in the p.m.f. partitioning, volume regulation, and stromal Ca(2+) and Mg(2+) dynamics. Patch-clamping of the intact envelope revealed both large-conductance porin-like channels, likely located in the outer envelope membrane and smaller conductance channels, more compatible with the inner envelope location. Possible equivalent model for the sandwich-like arrangement of the two envelope membranes within the patch electrode will be discussed, along with peculiar properties of the fast-activated cation channel in the context of the stromal pH control.
Keywords: ClC channel; cation channel; chloroplast envelope; magnesium; patch-clamp; porin; proton-motive force; thylakoid.
Figures
Similar articles
-
Transport Across Chloroplast Membranes: Optimizing Photosynthesis for Adverse Environmental Conditions.Mol Plant. 2016 Mar 7;9(3):356-370. doi: 10.1016/j.molp.2015.10.006. Epub 2015 Oct 24. Mol Plant. 2016. PMID: 26597501 Review.
-
Ion channels in the chloroplast envelope membrane.Biochemistry. 1995 Dec 12;34(49):15906-17. doi: 10.1021/bi00049a005. Biochemistry. 1995. PMID: 8519747
-
Lateral heterogeneity of the proton potential along the thylakoid membranes of chloroplasts.Biochim Biophys Acta Biomembr. 2017 Mar;1859(3):388-401. doi: 10.1016/j.bbamem.2016.11.016. Epub 2016 Dec 2. Biochim Biophys Acta Biomembr. 2017. PMID: 27916634
-
Regulation of photosynthesis by ion channels in cyanobacteria and higher plants.Biophys Chem. 2013 Dec 1;182:51-7. doi: 10.1016/j.bpc.2013.06.006. Epub 2013 Jun 29. Biophys Chem. 2013. PMID: 23891570 Review.
-
Fast-activating channel controls cation fluxes across the native chloroplast envelope.J Membr Biol. 2005 Apr;204(3):145-56. doi: 10.1007/s00232-005-0758-3. J Membr Biol. 2005. PMID: 16245037
Cited by
-
Transmembrane potential, an indicator in situ reporting cellular senescence and stress response in plant tissues.Plant Methods. 2023 Mar 21;19(1):27. doi: 10.1186/s13007-023-01006-0. Plant Methods. 2023. PMID: 36945027 Free PMC article.
-
Ion and metabolite transport in the chloroplast of algae: lessons from land plants.Cell Mol Life Sci. 2018 Jun;75(12):2153-2176. doi: 10.1007/s00018-018-2793-0. Epub 2018 Mar 14. Cell Mol Life Sci. 2018. PMID: 29541792 Free PMC article. Review.
-
Temperature sensitivity of carbon concentrating mechanisms in the diatom Phaeodactylum tricornutum.Photosynth Res. 2023 May;156(2):205-215. doi: 10.1007/s11120-023-01004-2. Epub 2023 Mar 7. Photosynth Res. 2023. PMID: 36881356 Free PMC article.
-
Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses.Plants (Basel). 2021 Jun 12;10(6):1202. doi: 10.3390/plants10061202. Plants (Basel). 2021. PMID: 34204700 Free PMC article.
-
Does a voltage-sensitive outer envelope transport mechanism contributes to the chloroplast iron uptake?Planta. 2016 Dec;244(6):1303-1313. doi: 10.1007/s00425-016-2586-3. Epub 2016 Aug 19. Planta. 2016. PMID: 27541495
References
-
- Allen G. A., Amtmann A., Sanders D. (1998). Calcium-dependent and calcium-independent K+ mobilization channels in Vicia faba guard cell vacuoles. J. Exp. Bot. 49, 305–318. 10.1093/jxb/49.Special_Issue.305 - DOI
-
- Allen J. F., Holmes N. G. (1986). Electron transport and redox titration, in Photosynthesis-Energy Transduction: A Practical Approach, eds Hipkins M. F., Baker N. R. (Oxford: IRL Press; ), 103–141.
-
- Becker D., Geiger D., Dunkel M., Roller A., Bertl A., Latz A., et al. . (2004). AtTPK4, an Arabidopsis tandem-pore K_ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 101, 15621–15626. 10.1073/pnas.0401502101 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

