Uninephrectomy in Rats on a Fixed Food Intake Potentiates Both Anorexia and Circulating Cytokine Subsets in Response to LPS
- PMID: 26734008
- PMCID: PMC4686617
- DOI: 10.3389/fimmu.2015.00641
Uninephrectomy in Rats on a Fixed Food Intake Potentiates Both Anorexia and Circulating Cytokine Subsets in Response to LPS
Abstract
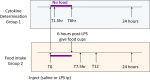
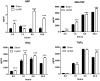
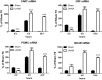
Recent human studies have suggested that mild reduction in kidney function can alter immune response and increase susceptibility to infection. The role of mild reduction in kidney function in altering susceptibility to bacterial lipopolysaccharide (LPS) responses was investigated in uninephrectomized rats compared to Sham-operated controls rats 4 weeks after surgery. Throughout the 4 weeks, all rats were maintained under mild food restriction at 90% of ad libitum intake to ensure the same caloric intake in both groups. In comparison to Sham, uninephrectomy (UniNX) potentiated LPS-induced anorexia by 2.1-fold. The circulating anorexigenic cytokines granulocyte-macrophage colony stimulating factor, interferon-γ, tumor necrosis factor-α, and complement-derived acylation-stimulating protein were elevated after LPS in UniNX animals compared to Sham animals. Interleukin(IL)1β and IL6 pro-inflammatory cytokines were transiently increased. Anti-inflammatory cytokines IL4 and IL10 did not differ or had a tendency to be lower in UniNX group compared to Sham animals. LPS-induced anorexia was associated with increased anorexigenic neuropeptides mRNA for pro-opiomelanocortin, corticotrophin-releasing factor, and cocaine-amphetamine-regulated transcript in the hypothalamus of both Sham and UniNX groups, but at higher levels in the UniNX group. Melanocortin-4-receptor mRNA was markedly increased in the UniNX group, which may have contributed to the enhanced anorexic response to LPS of the UniNX group. In summary, UniNX potentiates pro-inflammatory cytokine production, anorexia, and selected hypothalamic anorexigenic neuropeptides in response to LPS.
Keywords: LPS; Sprague-Dawley; anorexia; brain; cytokines; neuropeptides; rats; uninephrectomy.
Figures



References
-
- Ortega LM, Fornoni A. Role of cytokines in the pathogenesisof acute and chronic kidney disease, glomerulonephritis and end stage kidney disease. Int J Intereferon Cytokine Mediator Res (2010) 2:49–62.10.2147/IJICMR.S10111 - DOI
-
- Barsoum RS. Parasitic infections in organ transplantation. Exp Clin Transplant (2004) 2:258–67. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

