Synthesis of bi- and bis-1,2,3-triazoles by copper-catalyzed Huisgen cycloaddition: A family of valuable products by click chemistry
- PMID: 26734102
- PMCID: PMC4685768
- DOI: 10.3762/bjoc.11.276
Synthesis of bi- and bis-1,2,3-triazoles by copper-catalyzed Huisgen cycloaddition: A family of valuable products by click chemistry
Abstract
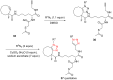
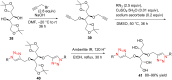
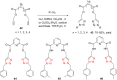
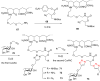
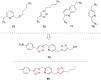
The Cu(I)-catalyzed azide-alkyne cycloaddition reaction, also known as click chemistry, has become a useful tool for the facile formation of 1,2,3-triazoles. Specifically, the utility of this reaction has been demonstrated by the synthesis of structurally diverse bi- and bis-1,2,3-triazoles. The present review focuses on the synthesis of such bi- and bistriazoles and the importance of using copper-promoted click chemistry (CuAAC) for such transformations. In addition, the application of bitriazoles and the related CuAAAC reaction in different fields, including medicinal chemistry, coordination chemistry, biochemistry, and supramolecular chemistry, have been highlighted.
Keywords: bistriazoles; click chemistry; cycloaddition; homogeneous catalysis; oxidative coupling.
Figures
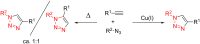

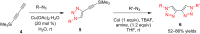
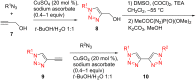
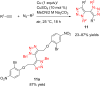
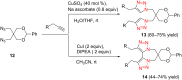
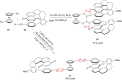
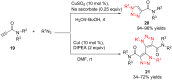
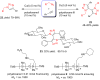
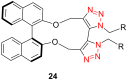
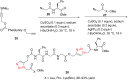
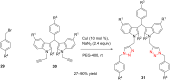
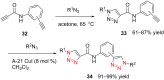
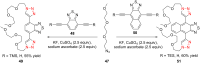
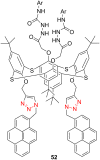

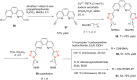
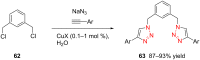
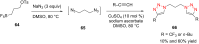
References
-
- Huisgen R. Pure Appl Chem. 1989;61:613–628. doi: 10.1351/pac198961040613. - DOI
-
- Huisgen R, Szeimies G, Möbius L. Chem Ber. 1967;100:2494–2507. doi: 10.1002/cber.19671000806. - DOI
-
- Bastide J, Hamelin J, Texier F, Ven V Q. Bull Soc Chim Fr. 1973:2555–2579.
-
- Bastide J, Hamelin J, Texier F, Ven V Q. Bull Soc Chim Fr. 1973:2871–2887.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
