The selenium content of SEPP1 versus selenium requirements in vertebrates
- PMID: 26734501
- PMCID: PMC4699779
- DOI: 10.7717/peerj.1244
The selenium content of SEPP1 versus selenium requirements in vertebrates
Abstract
Selenoprotein P (SEPP1) distributes selenium (Se) throughout the body via the circulatory system. For vertebrates, the Se content of SEPP1 varies from 7 to 18 Se atoms depending on the species, but the reason for this variation remains unclear. Herein we provide evidence that vertebrate SEPP1 Sec content correlates positively with Se requirements. As the Se content of full length SEPP1 is genetically determined, this presents a unique case where a nutrient requirement can be predicted based on genomic sequence information.
Keywords: Nutrition; SEPP1; Selenium requirements; Selenoprotein; Selenoprotein P.
Conflict of interest statement
Kristin Hamre is an Academic Editor for PeerJ. Sam Penglase is an employee of Aquaculture Research Solutions, Mundingburra, QLD
Figures
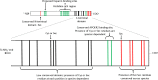

 ), but unannotated genomes. SEPP1 Sec content in these fish were assumed to be within the likely range of 15–17 Sec residues found for fish in general (5PL Asymmetric sigmoidal, R2 = 0.86, y = − 9.98 + (26.9/((1 + 10((−2.23397 − X) × 4.661))1.910)). Shaded boxes group animals within classes. The X axis is log transformed.
), but unannotated genomes. SEPP1 Sec content in these fish were assumed to be within the likely range of 15–17 Sec residues found for fish in general (5PL Asymmetric sigmoidal, R2 = 0.86, y = − 9.98 + (26.9/((1 + 10((−2.23397 − X) × 4.661))1.910)). Shaded boxes group animals within classes. The X axis is log transformed.Similar articles
-
Deletion of selenoprotein P upregulates urinary selenium excretion and depresses whole-body selenium content.Biochim Biophys Acta. 2006 Dec;1760(12):1789-93. doi: 10.1016/j.bbagen.2006.08.010. Epub 2006 Aug 18. Biochim Biophys Acta. 2006. PMID: 17014962 Free PMC article.
-
Isoform-specific binding of selenoprotein P to the β-propeller domain of apolipoprotein E receptor 2 mediates selenium supply.J Biol Chem. 2014 Mar 28;289(13):9195-207. doi: 10.1074/jbc.M114.549014. Epub 2014 Feb 13. J Biol Chem. 2014. PMID: 24532792 Free PMC article.
-
Biomonitoring of selenoprotein P in human serum by fast affinity chromatography coupled to ICP-MS.Int J Hyg Environ Health. 2018 Apr;221(3):564-568. doi: 10.1016/j.ijheh.2018.02.006. Epub 2018 Feb 19. Int J Hyg Environ Health. 2018. PMID: 29478805
-
Selenoprotein P-expression, functions, and roles in mammals.Biochim Biophys Acta. 2009 Nov;1790(11):1441-7. doi: 10.1016/j.bbagen.2009.03.026. Epub 2009 Apr 1. Biochim Biophys Acta. 2009. PMID: 19345254 Free PMC article. Review.
-
Regulation of Selenium Metabolism and Transport.Annu Rev Nutr. 2015;35:109-34. doi: 10.1146/annurev-nutr-071714-034250. Epub 2015 May 13. Annu Rev Nutr. 2015. PMID: 25974694 Review.
Cited by
-
Nutrition and Metabolism of Minerals in Fish.Animals (Basel). 2021 Sep 16;11(9):2711. doi: 10.3390/ani11092711. Animals (Basel). 2021. PMID: 34573676 Free PMC article. Review.
-
Advances in selenium supplementation: From selenium-enriched yeast to potential selenium-enriched insects, and selenium nanoparticles.Anim Nutr. 2023 May 20;14:193-203. doi: 10.1016/j.aninu.2023.05.002. eCollection 2023 Sep. Anim Nutr. 2023. PMID: 37484993 Free PMC article. Review.
-
Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated ELISA for selenoprotein P.Redox Biol. 2017 Apr;11:403-414. doi: 10.1016/j.redox.2016.12.025. Epub 2016 Dec 29. Redox Biol. 2017. PMID: 28064116 Free PMC article.
References
-
- Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, Winfrey VP, Austin LM. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. Journal of Neuroscience. 2007;27(23):6207–6211. doi: 10.1523/JNEUROSCI.1153-07.2007. - DOI - PMC - PubMed
-
- Chiu-Ugalde J, Theilig F, Behrends T, Drebes J, Sieland C, Subbarayal P, Köhrle J, Hammes A, Schomburg L, Schweizer U. Mutation of megalin leads to urinary loss of selenoprotein P and selenium deficiency in serum, liver, kidneys and brain. The Biochemical Journal. 2010;431(1):103–111. doi: 10.1042/BJ20100779. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

