Hypomyelination following deletion of Tsc2 in oligodendrocyte precursors
- PMID: 26734657
- PMCID: PMC4693589
- DOI: 10.1002/acn3.254
Hypomyelination following deletion of Tsc2 in oligodendrocyte precursors
Abstract
Objective: While abnormalities in myelin in tuberous sclerosis complex (TSC) have been known for some time, recent imaging-based data suggest myelin abnormalities may be independent of the pathognomonic cortical lesions ("tubers"). Multiple mouse models of TSC exhibit myelination deficits, though the cell types responsible and the mechanisms underlying the myelin abnormalities remain unclear.
Methods: To determine the role of alterations in mTOR signaling in myelination, we generated a conditional knockout (CKO) mouse model using Cre-recombinase and the Olig2 promoter to inactivate the Tsc2 gene in oligodendrocyte precursor cells.
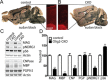
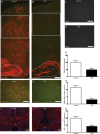
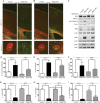
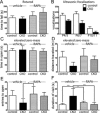
Results: Characterization of myelin and myelin constituent proteins demonstrated a marked hypomyelination phenotype. Diffusion-based magnetic resonance imaging studies were likewise consistent with hypomyelination. Hypomyelination was due in part to decreased myelinated axon density and myelin thickness as well as decreased oligodendrocyte numbers. Coincident with hypomyelination, an extensive gliosis was seen in both the cortex and white matter tracks, suggesting alterations in cell fate due to changes in mTOR activity in oligodendrocyte precursors. Despite a high-frequency appendicular tremor and altered gait in CKO mice, no significant changes in activity, vocalizations, or anxiety-like phenotypes were seen.
Interpretation: Our findings support a known role of mTOR signaling in regulation of myelination and demonstrate that increased mTORC1 activity early in development within oligodendrocytes results in hypomyelination and not hypermyelination. Our data further support a dissociation between decreased Akt activity and increased mTORC1 activity toward hypomyelination. Thus, therapies promoting activation of Akt-dependent pathways while reducing mTORC1 activity may prove beneficial in treatment of human disease.
Figures



References
-
- Arulrajah S, Ertan G, Jordan L, et al. Magnetic resonance imaging and diffusion‐weighted imaging of normal‐appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology 2009;51:781–786. - PubMed
-
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 1996;111:209–219. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

