Neuroprotective effects of deep hypothermia in refractory status epilepticus
- PMID: 26734661
- PMCID: PMC4693587
- DOI: 10.1002/acn3.262
Neuroprotective effects of deep hypothermia in refractory status epilepticus
Abstract
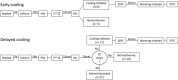
Objective: Pharmacoresistance develops quickly during repetitive seizures, and refractory status epilepticus (RSE) remains a therapeutic challenge. The outcome of RSE is poor, with high mortality and morbidity. New treatments are needed. Deep hypothermia (20°C) is used clinically during reconstructive cardiac surgery and neurosurgery, and has proved safe and effective in those indications. We tested the hypothesis that deep hypothermia reduces RSE and its long-term consequences.
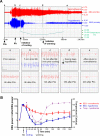
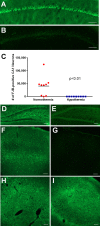
Methods: We used a model of SE induced by lithium and pilocarpine and refractory to midazolam. Several EEG measures were recorded in both hypothermic (n = 17) and normothermic (n = 20) animals. Neuronal injury (by Fluoro-Jade B), cell-mediated inflammation, and breakdown of the blood-brain barrier (BBB) (by immunohistochemistry) were studied 48 h following SE onset.
Results: Normothermic rats in RSE seized for 4.1 ± 1.1 h, and at 48 h they displayed extensive neuronal injury in many brain regions, including hippocampus, dentate gyrus, amygdala, entorhinal and pyriform cortices, thalamus, caudate/putamen, and the frontoparietal neocortex. Deep hypothermia (20°C) of 30 min duration terminated RSE within 12 min of initiation of hypothermia, reduced EEG power and seizure activity upon rewarming, and eliminated SE-induced neuronal injury in most animals. Normothermic rats showed widespread breakdown of the BBB, and extensive macrophage infiltration in areas of neuronal injury, which were completely absent in animals treated with hypothermia.
Interpretation: These results suggest that deep hypothermia may open a new therapeutic avenue for the treatment of RSE and for the prevention of its long-term consequences.
Figures

References
-
- DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population‐based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46:1029–1035. - PubMed
-
- Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population‐based study. Epilepsia 2001;42:714–718. - PubMed
-
- Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–798. - PubMed
-
- Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia 1994;35:27–34. - PubMed
-
- Fountain NB. Status epilepticus: risk factors and complications. Epilepsia 2000;41(Suppl 2):S23–S30. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

