Efficacy and safety of a single dose pentamidine (7mg/kg) for patients with cutaneous leishmaniasis caused by L. guyanensis: a pilot study
- PMID: 26734860
- PMCID: PMC4689067
- DOI: 10.1590/abd1806-4841.20153956
Efficacy and safety of a single dose pentamidine (7mg/kg) for patients with cutaneous leishmaniasis caused by L. guyanensis: a pilot study
Abstract
Background: There have been few studies on pentamidine in the Americas; and there is no consensus regarding the dose that should be applied.
Objectives: To evaluate the use of pentamidine in a single dose to treat cutaneous leishmaniasis.
Methods: Clinical trial of phase II pilot study with 20 patients. Pentamidine was used at a dose of 7 mg/kg, in a single dose. Safety and adverse effects were also assessed. Patients were reviewed one, two, and six months after the end of treatments.
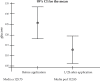
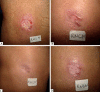
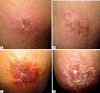
Results: there was no difference between the treatment groups in relation to gender, age, number or location of the lesions. Pentamidine, applied in a single dose, obtained an effectiveness of 55%. Mild adverse events were reported by 17 (85%) patients, mainly transient pain at the site of applications (85%), while nausea (5%), malaise (5%) and dizziness (5%) were reported in one patient. No patient had sterile abscess after taking medication at a single dose of 7mg/kg.
Conclusions: Clinical studies with larger samples of patients would enable a better clinical response of pent amidine at a single dose of 7mg, allowing the application of more powerful statistical tests, thus providing more evidences of the decrease in the effectiveness of that medication. Hence, it is important to have larger studies with new diagrams and/or new medications.
Conflict of interest statement
Conflict of Interest: None
Figures
References
-
- World Health Organization . Sixtieth World Health Assembly. Control of leishmaniasis. World Health Organization,; Geneva, Switzerland: 2007. [2012 Jun 14]. Available from: http://www.who.int/neglected_diseases/mediacentre/WHA_60.13_Eng.pdf.
-
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. - PubMed
-
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Manual de Vigilância da Leishmaniose Tegumentar Americana. 2. ed. Brasília: Editora do Ministério da Saúde; 2007. pp. 182–182. Série A. Normas e Manuais Técnicos.
-
- Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, da Silva RM, Gadelha Yamashita EP, de Oliveira Penna G, et al. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis Caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg. 2011;84:255–260. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
