Circulating microRNA-based screening tool for breast cancer
- PMID: 26734993
- PMCID: PMC4868695
- DOI: 10.18632/oncotarget.6786
Circulating microRNA-based screening tool for breast cancer
Abstract
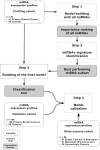
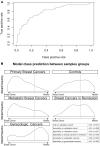
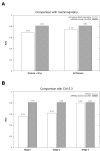
Circulating microRNAs (miRNAs) are increasingly recognized as powerful biomarkers in several pathologies, including breast cancer. Here, their plasmatic levels were measured to be used as an alternative screening procedure to mammography for breast cancer diagnosis.A plasma miRNA profile was determined by RT-qPCR in a cohort of 378 women. A diagnostic model was designed based on the expression of 8 miRNAs measured first in a profiling cohort composed of 41 primary breast cancers and 45 controls, and further validated in diverse cohorts composed of 108 primary breast cancers, 88 controls, 35 breast cancers in remission, 31 metastatic breast cancers and 30 gynecologic tumors.A receiver operating characteristic curve derived from the 8-miRNA random forest based diagnostic tool exhibited an area under the curve of 0.81. The accuracy of the diagnostic tool remained unchanged considering age and tumor stage. The miRNA signature correctly identified patients with metastatic breast cancer. The use of the classification model on cohorts of patients with breast cancers in remission and with gynecologic cancers yielded prediction distributions similar to that of the control group.Using a multivariate supervised learning method and a set of 8 circulating miRNAs, we designed an accurate, minimally invasive screening tool for breast cancer.
Keywords: biomarkers; breast cancer; circulating microRNAs; minimally invasive screening.
Conflict of interest statement
None.
Figures

References
-
- Jemal A, Bray F, Center Melissa M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Bleyer A, Welch HG. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. N Engl J Med. 2012;367:1998–2005. - PubMed
-
- Kohn L, Mambourg F, Robays J, Albertijn M, Janssens J, Hoefnagels K, Ronsmans M, Jonckheer P. Good Clinical Practice (GCP) Brussels: Belgian Health Care Knowledge Centre (KCE); 2014. Informed choice on breast cancer screening: messages to support informed decision. KCE Reports 216. D/2014/10.273/03.
-
- Eulalio A, Huntzinger E, Izaurralde E. Getting to the Root of miRNA-Mediated Gene Silencing. Cell. 2008;132:9–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

