The functional diversity of retinal ganglion cells in the mouse
- PMID: 26735013
- PMCID: PMC4724341
- DOI: 10.1038/nature16468
The functional diversity of retinal ganglion cells in the mouse
Abstract
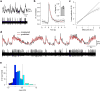
In the vertebrate visual system, all output of the retina is carried by retinal ganglion cells. Each type encodes distinct visual features in parallel for transmission to the brain. How many such 'output channels' exist and what each encodes are areas of intense debate. In the mouse, anatomical estimates range from 15 to 20 channels, and only a handful are functionally understood. By combining two-photon calcium imaging to obtain dense retinal recordings and unsupervised clustering of the resulting sample of more than 11,000 cells, here we show that the mouse retina harbours substantially more than 30 functional output channels. These include all known and several new ganglion cell types, as verified by genetic and anatomical criteria. Therefore, information channels from the mouse eye to the mouse brain are considerably more diverse than shown thus far by anatomical studies, suggesting an encoding strategy resembling that used in state-of-the-art artificial vision systems.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






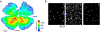





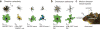

References
-
- Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci. 2014;15:507–19. - PubMed
-
- Lettvin J, Maturana H, McCulloch W, Pitts W. What the Frog’s Eye Tells the Frog’s Brain. Proc IRE. 1959;47:1940–1951.
-
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II Intracellular recording. J Neurophysiol. 1969;32:339–355. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

