Asymmetric Syntheses of the Flavonoid Diels-Alder Natural Products Sanggenons C and O
- PMID: 26735066
- PMCID: PMC4863937
- DOI: 10.1021/jacs.5b12778
Asymmetric Syntheses of the Flavonoid Diels-Alder Natural Products Sanggenons C and O
Abstract
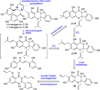
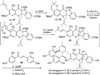
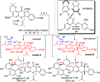
Metal-catalyzed, double Claisen rearrangement of a bis-allyloxyflavone has been utilized to enable a concise synthesis of the hydrobenzofuro[3,2-b]chromenone core structure of the natural products sanggenon A and sanggenol F. In addition, catalytic, enantioselective [4+2] cycloadditions of 2'-hydroxychalcones have been accomplished using B(OPh)3/BINOL complexes. Asymmetric syntheses of the flavonoid Diels-Alder natural products sanggenons C and O have been achieved employing a stereodivergent reaction of a racemic mixture (stereodivergent RRM) involving [4+2] cycloaddition.
Figures

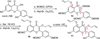


References
-
- Fukai T, Pei Y-H, Nomura T, Xu C-Q, Wu L-J, Chen Y-J. Phytochemistry. 1998;47:273.
- Shen R-C, Lin M. Phytochemistry. 2001;57:1231. - PubMed
- Shi Y-Q, Fukai T, Sakagami H, Chang W-J, Yang P-Q, Wang F-P, Nomura T. J. Nat. Prod. 2001;64:181. - PubMed
- Rollinger JM, Bodensieck A, Seger C, Ellmerer E, Bauer R, Langer T, Stuppner H. Planta Med. 2005;71:399. - PubMed
-
- Nomura T, Fukai T, Hano Y, Uzawa J. Heterocycles. 1981;16:2141.
- Shi YQ, Fukai T, Ochiai M, Nomura T. Heterocycles. 2001;55:13.
-
- Hano Y, Suzuki S, Nomura T, Itaka Y. Heterocycles. 1988;27:2315.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

