Combating autophagy is a strategy to increase cytotoxic effects of novel ALK inhibitor entrectinib in neuroblastoma cells
- PMID: 26735175
- PMCID: PMC4868711
- DOI: 10.18632/oncotarget.6778
Combating autophagy is a strategy to increase cytotoxic effects of novel ALK inhibitor entrectinib in neuroblastoma cells
Abstract
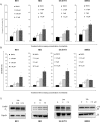
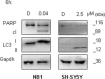
Neuroblastoma (NB) is a threatening childhood malignancy. Its prognosis is affected by several morphological, and biological characteristics, including the constitutive expression of ALK tyrosine kinase. In this study we examined the therapeutic potential of a novel ALK inhibitor, entrectinib, in obliterating NB tumor cells. Entrectinib showed the growth-inhibitory effects on NB cells with a 50% inhibitory concentration range of 0.03-5 μM. In the ALK-dependent cells, entrectinib mediated G1-arrest, which was associated with modified expression of multiple cell-cycle regulators. Down-regulation of Ki-67, and attenuated phosphorylation of ERK1/2, and STAT3, correlated with observed antiproliferative capacity of entrectinib. Initial cytostatic activity of entrectinib was followed by concentration-dependent apoptotic cell death, and Caspase-3 activation. However, we delineated a reduced sensitivity of ALK mutated NB cells to entrectinib, and demonstrated strong activation of autophagy in SH-SY5YF1174L NB cell line. Abrogation of autophagy by chloroquine increased significantly the toxicity of entrectinib, as confirmed by enhanced death rate, and PARP protein cleavage in SH-SY5YF1174L cells. In aggregate, our data show that entrectinib inhibits proliferation, and induces G1-arrest, and apoptosis in NB cells. We propose entrectinib for further consideration in treatment of NB, and recommend pharmacological inhibition of autophagy to be explored for a combined therapeutic approach in NB patients that might develop resistance to entrectinib.
Keywords: ALK inhibitors; autophagy; drug combination; neuroblastoma.
Conflict of interest statement
The authors have no potential conflict of interest to declare.
Figures







Similar articles
-
Entrectinib is a potent inhibitor of Trk-driven neuroblastomas in a xenograft mouse model.Cancer Lett. 2016 Mar 28;372(2):179-86. doi: 10.1016/j.canlet.2016.01.018. Epub 2016 Jan 18. Cancer Lett. 2016. PMID: 26797418 Free PMC article.
-
Novel ALK inhibitor AZD3463 inhibits neuroblastoma growth by overcoming crizotinib resistance and inducing apoptosis.Sci Rep. 2016 Jan 20;6:19423. doi: 10.1038/srep19423. Sci Rep. 2016. PMID: 26786851 Free PMC article.
-
Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma.Drug Des Devel Ther. 2018 Oct 23;12:3549-3561. doi: 10.2147/DDDT.S147384. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30425456 Free PMC article. Review.
-
Mechanisms of Entrectinib Resistance in a Neuroblastoma Xenograft Model.Mol Cancer Ther. 2020 Mar;19(3):920-926. doi: 10.1158/1535-7163.MCT-18-1044. Epub 2019 Dec 23. Mol Cancer Ther. 2020. PMID: 31871269
-
Entrectinib: a potent new TRK, ROS1, and ALK inhibitor.Expert Opin Investig Drugs. 2015;24(11):1493-500. doi: 10.1517/13543784.2015.1096344. Epub 2015 Oct 12. Expert Opin Investig Drugs. 2015. PMID: 26457764 Review.
Cited by
-
GD2 ganglioside-binding antibody 14G2a and specific aurora A kinase inhibitor MK-5108 induce autophagy in IMR-32 neuroblastoma cells.Apoptosis. 2018 Oct;23(9-10):492-511. doi: 10.1007/s10495-018-1472-9. Apoptosis. 2018. PMID: 30027525 Free PMC article.
-
Molecularly Targeted Therapy for Neuroblastoma.Children (Basel). 2018 Oct 15;5(10):142. doi: 10.3390/children5100142. Children (Basel). 2018. PMID: 30326621 Free PMC article. Review.
-
Is Autophagy Targeting a Valid Adjuvant Strategy in Conjunction with Tyrosine Kinase Inhibitors?Cancers (Basel). 2024 Aug 28;16(17):2989. doi: 10.3390/cancers16172989. Cancers (Basel). 2024. PMID: 39272847 Free PMC article. Review.
-
Interplay of autophagy, receptor tyrosine kinase signalling and endocytic trafficking.Essays Biochem. 2017 Dec 12;61(6):597-607. doi: 10.1042/EBC20170091. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233871 Free PMC article. Review.
-
Cell death-based treatment of neuroblastoma.Cell Death Dis. 2018 Jan 25;9(2):113. doi: 10.1038/s41419-017-0060-1. Cell Death Dis. 2018. PMID: 29371588 Free PMC article. Review.
References
-
- SEER Program (National Cancer Institute (U.S.)) and National Cancer Institute (U.S.) 1999 Cancer incidence and survival among children and adolescents United States SEER Program, 1975-1995. Bethesda, Md: National Cancer Institute;
-
- Tonini GP, Nakagawara A, Berthold F. Towards a turning point of neuroblastoma therapy. Cancer Lett. 2012;326:128–134. - PubMed
-
- Schwab M. MYCN in neuronal tumours. Cancer Lett. 2004;204:179–187. - PubMed
-
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

