GNA13 as a prognostic factor and mediator of gastric cancer progression
- PMID: 26735177
- PMCID: PMC4826215
- DOI: 10.18632/oncotarget.6780
GNA13 as a prognostic factor and mediator of gastric cancer progression
Abstract
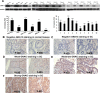
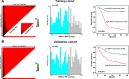
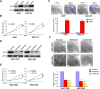
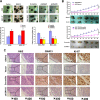
Guanine nucleotide binding protein (G protein), alpha 13 (GNA13) has been implicated as an oncogenic protein in several human cancers. In this study, GNA13 was characterized for its role in gastric cancer (GC) progression and underlying molecular mechanisms. The expression dynamics of GNA13 were examined by immunohistochemistry (IHC) in two independent cohorts of GC samples. A series of in-vivo and in-vitro assays was performed to elucidate the function of GNA13 in GC and its underlying mechanisms. In both two cohorts of GC samples, we observed that GNA13 was markedly overexpressed in GC tissues and associated closely with aggressive magnitude of GC progression and poor patients' survival. Further study showed that upregulation of GNA13 expression increased the proliferation and tumorigenicity of GC cells in vitro and in vivo, by promoting cell growth rate, colony formation, and tumor formation in nude mice. By contrast, knockdown of GNA13 effectively suppressed the proliferation and tumorigenicity of GC cells in vitro and in vivo. Our results also demonstrated that the molecular mechanisms of the effect of GNA13 in GC included promotion of G1/S cell cycle transition through upregulation of c-Myc, activation of AKT and ERK activity, suppression of FOXO1 activity, upregulation of cyclin-dependent kinase (CDK) regulator cyclin D1 and downregulation of CDK inhibitor p21Cip1 and p27Kip1. Our present study illustrated that GNA13 has an important role in promoting proliferation and tumorigenicity of GC, and may represent a novel prognostic biomarker and therapeutic target for this disease.
Keywords: GNA13; gastric cancer; proliferation; tumorigenicity.
Conflict of interest statement
No conflicts of interest were declared
Figures


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA. 2011;61:69–90. - PubMed
-
- Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S, Group JS. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. International journal of cancer. 2006;118:2315–2321. - PubMed
-
- Coupland VH, Lagergren J, Luchtenborg M, Jack RH, Allum W, Holmberg L, Hanna GB, Pearce N, Moller H. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004–2008. Gut. 2013;62:961–966. - PubMed
-
- Chen CN, Lin JJ, Chen JJ, Lee PH, Yang CY, Kuo ML, Chang KJ, Hsieh FJ. Gene expression profile predicts patient survival of gastric cancer after surgical resection. Journal of clinical oncology. 2005;23:7286–7295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

