NG2 proteoglycan as a pericyte target for anticancer therapy by tumor vessel infarction with retargeted tissue factor
- PMID: 26735180
- PMCID: PMC4872748
- DOI: 10.18632/oncotarget.6725
NG2 proteoglycan as a pericyte target for anticancer therapy by tumor vessel infarction with retargeted tissue factor
Abstract
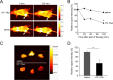
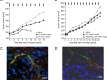
tTF-TAA and tTF-LTL are fusion proteins consisting of the extracellular domain of tissue factor (TF) and the peptides TAASGVRSMH and LTLRWVGLMS, respectively. These peptides represent ligands of NG2, a surface proteoglycan expressed on angiogenic pericytes and some tumor cells. Here we have expressed the model compound tTF-NGR, tTF-TAA, and tTF-LTL with different lengths in the TF domain in E. coli and used these fusion proteins for functional studies in anticancer therapy. We aimed to retarget TF to tumor vessels leading to tumor vessel infarction with two barriers of selectivity, a) the leaky endothelial lining in tumor vessels with the target NG2 being expressed on pericytes on the abluminal side of the endothelial cell barrier and b) the preferential expression of NG2 on angiogenic vessels such as in tumors. Chromatography-purified tTF-TAA showed identical Factor X (FX)-activating procoagulatory activity as the model compound tTF-NGR with Km values of approx. 0.15 nM in Michaelis-Menten kinetics. The procoagulatory activity of tTF-LTL varied with the chosen length of the TF part of the fusion protein. Flow cytometry revealed specific binding of tTF-TAA to NG2-expressing pericytes and tumor cells with low affinity and dissociation KD in the high nM range. In vivo and ex vivo fluorescence imaging of tumor xenograft-carrying animals and of the explanted tumors showed reduction of tumor blood flow upon tTF-TAA application. Therapeutic experiments showed a reproducible antitumor activity of tTF-TAA against NG2-expressing A549-tumor xenografts, however, with a rather small therapeutic window (active/toxic dose in mg/kg body weight).
Keywords: NG2 proteoglycan; cancer; truncated tissue factor; vascular infarction; vascular targeting.
Conflict of interest statement
R.M. M. and W.E. B. share a patent on vascular targeting with TF constructs. No potential conflicts of interest was declared by the other authors.
Figures







References
-
- Folkman J. Angiogenesis in cancer, vascular, reumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
-
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. - PubMed
-
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. - PubMed
-
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

