A Shift from Cellular to Humoral Responses Contributes to Innate Immune Memory in the Vector Snail Biomphalaria glabrata
- PMID: 26735307
- PMCID: PMC4703209
- DOI: 10.1371/journal.ppat.1005361
A Shift from Cellular to Humoral Responses Contributes to Innate Immune Memory in the Vector Snail Biomphalaria glabrata
Abstract
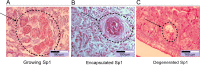
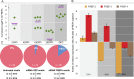
Discoveries made over the past ten years have provided evidence that invertebrate antiparasitic responses may be primed in a sustainable manner, leading to the failure of a secondary encounter with the same pathogen. This phenomenon called "immune priming" or "innate immune memory" was mainly phenomenological. The demonstration of this process remains to be obtained and the underlying mechanisms remain to be discovered and exhaustively tested with rigorous functional and molecular methods, to eliminate all alternative explanations. In order to achieve this ambitious aim, the present study focuses on the Lophotrochozoan snail, Biomphalaria glabrata, in which innate immune memory was recently reported. We provide herein the first evidence that a shift from a cellular immune response (encapsulation) to a humoral immune response (biomphalysin) occurs during the development of innate memory. The molecular characterisation of this process in Biomphalaria/Schistosoma system was undertaken to reconcile mechanisms with phenomena, opening the way to a better comprehension of innate immune memory in invertebrates. This prompted us to revisit the artificial dichotomy between innate and memory immunity in invertebrate systems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
A Novel Mechanism of Immune Memory Unveiled at the Invertebrate-Parasite Interface.Trends Parasitol. 2016 May;32(5):353-355. doi: 10.1016/j.pt.2016.02.005. Epub 2016 Mar 4. Trends Parasitol. 2016. PMID: 26953517
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

