Development of a Screening Tool for Predicting Adverse Outcomes of Gestational Diabetes Mellitus: A Retrospective Cohort Study
- PMID: 26735528
- PMCID: PMC4706248
- DOI: 10.1097/MD.0000000000002204
Development of a Screening Tool for Predicting Adverse Outcomes of Gestational Diabetes Mellitus: A Retrospective Cohort Study
Abstract
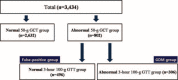
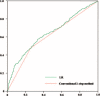
Gestational diabetes mellitus (GDM) is a common disease in pregnancy causing maternal and fetal complications. To prevent these adverse outcomes, optimal screening and diagnostic criteria must be adequate, timely, and efficient. This study suggests a novel approach that is practical, efficient, and patient- and clinician-friendly in predicting adverse outcomes of GDM. The authors conducted a retrospective cohort study via medical record review of patients admitted between March 2001 and April 2013 at the Severance Hospital, Seoul, South Korea. Patients diagnosed by a conventional 2-step method were evaluated according to the presence of adverse outcomes (neonatal hypoglycemia, hyperbilirubinemia, and hyperinsulinemia; admission to the neonatal intensive care unit; large for gestational age; gestational insulin therapy; and gestational hypertension). Of 802 women who had an abnormal 50-g, 1-hour glucose challenge test, 306 were diagnosed with GDM and 496 did not have GDM (false-positive group). In the GDM group, 218 women (71.2%) had adverse outcomes. In contrast, 240 women (48.4%) in the false-positive group had adverse outcomes. Women with adverse outcomes had a significantly higher body mass index (BMI) at entry (P = 0.03) and fasting blood glucose (FBG) (P = 0.03). Our logistic regression model derived from 2 variables, BMI at entry and FBG, predicted GDM adverse outcome with an area under the curve of 0.642, accuracy of 61.3%, sensitivity of 57.2%, and specificity of 66.9% compared with the conventional 2-step method with an area under the curve of 0.610, accuracy of 59.1%, sensitivity of 47.6%, and specificity of 74.4%. Our model performed better in predicting GDM adverse outcomes than the conventional 2-step method using only BMI at entry and FBG. Moreover, our model represents a practical, inexpensive, efficient, reproducible, easy, and patient- and clinician-friendly approach.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Committee on Practice Bulletins: Obstetrics. Practice Bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol 2013; 122:406–416. - PubMed
-
- Coustan DR. Harris MI, Cowie CC, Stern MP, et al. Gestational diabetes. Diabetes in America 2nd ed.Baltimore, MD: National Institutes of Health; 1995. 703–717.
-
- Sermer M, Naylor CD, Gare DJ, et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto Tri-Hospital Gestational Diabetes Project. Am J Obstet Gynecol 1995; 173:146–156. - PubMed
-
- Dornhorst A, Rossi M. Risk and prevention of type 2 diabetes in women with gestational diabetes. Diabetes Care 1998; 21:B43–B49. - PubMed
-
- Petitt DJ, Bennett PH, Knowler WC, et al. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy. Long-term effects on obesity and glucose tolerance in the offspring. Diabetes 1985; 34:119–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

