Computer Tomography Imaging Findings of Abdominal Follicular Dendritic Cell Sarcoma: A Report of 5 Cases
- PMID: 26735543
- PMCID: PMC4706263
- DOI: 10.1097/MD.0000000000002404
Computer Tomography Imaging Findings of Abdominal Follicular Dendritic Cell Sarcoma: A Report of 5 Cases
Abstract
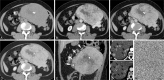
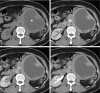
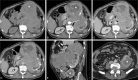
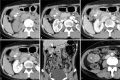
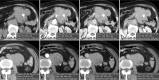
Follicular dendritic cell sarcoma (FDCS) is a neoplasm that arises from follicular dendritic cells. FDCSs originating in the abdomen are extremely rare. Clinically, they often mimic a wide variety of other abdominal tumors, and correct preoperative diagnosis is often a challenging task. To date, only scattered cases of abdominal FDCS have been reported and few data are available on their radiological features. Here we present the computer tomography imaging findings of 5 patients with surgically and pathologically demonstrated abdominal FDCS. An abdominal FDCS should be included in the differential diagnosis when single or multiple masses with relatively large size, well- or ill-defined borders, complex internal architecture with marked internal necrosis and/or focal calcification, and heterogeneous enhancement with "rapid wash-in and slow wash-out" or "progressive enhancement" enhancement patterns in the solid component are seen.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Intra-abdominal Follicular Dendritic Cell Sarcoma (FDCS): Series of 18 cases of a rare entity from Pakistan.Ann Diagn Pathol. 2020 Dec;49:151595. doi: 10.1016/j.anndiagpath.2020.151595. Epub 2020 Aug 15. Ann Diagn Pathol. 2020. PMID: 32905993
-
Imaging features and radiologic-pathologic correlations of inflammatory pseudotumor-like follicular dendritic cell sarcoma.BMC Med Imaging. 2021 Mar 17;21(1):52. doi: 10.1186/s12880-021-00584-6. BMC Med Imaging. 2021. PMID: 33731032 Free PMC article.
-
Transformation of Castleman's disease into follicular dendritic cell sarcoma, presenting as an asymptomatic intra-abdominal mass.Korean J Gastroenterol. 2013 Aug 25;62(2):131-4. doi: 10.4166/kjg.2013.62.2.131. Korean J Gastroenterol. 2013. PMID: 23981949
-
Clinicopathologic profile of intra-abdominal follicular dendritic cell sarcoma: A study of three cases with a literature review.Indian J Pathol Microbiol. 2024 Jan-Mar;67(1):195-200. doi: 10.4103/ijpm.ijpm_1089_21. Indian J Pathol Microbiol. 2024. PMID: 38358221 Review.
-
Mediastinal follicular dendritic cell sarcoma: a rare, potentially under-recognized, and often misdiagnosed disease.Diagn Pathol. 2019 Jan 15;14(1):5. doi: 10.1186/s13000-019-0779-3. Diagn Pathol. 2019. PMID: 30646936 Free PMC article. Review.
Cited by
-
Follicular dendritic cell sarcoma arising in the stomach and abdominal cavity: A case report.Medicine (Baltimore). 2023 Aug 4;102(31):e34289. doi: 10.1097/MD.0000000000034289. Medicine (Baltimore). 2023. PMID: 37543831 Free PMC article.
-
Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumors of the liver: Two case reports and literature review.World J Gastroenterol. 2019 Dec 7;25(45):6693-6703. doi: 10.3748/wjg.v25.i45.6693. World J Gastroenterol. 2019. PMID: 31832007 Free PMC article. Review.
-
Follicular Dendritic Cell Sarcoma of Gastrointestinal Tract: an Uncommon Lesion, Commonly Missed.J Gastrointest Cancer. 2019 Dec;50(4):913-918. doi: 10.1007/s12029-018-0178-0. J Gastrointest Cancer. 2019. PMID: 30430359
-
Follicular dendritic cell sarcoma of the stomach in a young male: A rare case report and literature review.Medicine (Baltimore). 2025 Jul 4;104(27):e43004. doi: 10.1097/MD.0000000000043004. Medicine (Baltimore). 2025. PMID: 40629639 Free PMC article. Review.
-
Paraneoplastic pemphigus and myasthenia gravis as the first manifestations of a rare case of pancreatic follicular dendritic cell sarcoma: CT findings and review of literature.BMC Gastroenterol. 2019 Jun 14;19(1):92. doi: 10.1186/s12876-019-1008-y. BMC Gastroenterol. 2019. PMID: 31200650 Free PMC article. Review.
References
-
- Tew JG, Kosco MH, Burton GF, et al. Follicular dendritic cells as accessory cells. Immunol Rev 1990; 117:185–211. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

