Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea
- PMID: 26735992
- PMCID: PMC5856332
- DOI: 10.1056/NEJMoa1511812
Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea
Abstract
Background: In the wake of the recent outbreak of Ebola virus disease (EVD) in several African countries, the World Health Organization prioritized the evaluation of treatment with convalescent plasma derived from patients who have recovered from the disease. We evaluated the safety and efficacy of convalescent plasma for the treatment of EVD in Guinea.
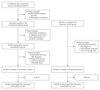
Methods: In this nonrandomized, comparative study, 99 patients of various ages (including pregnant women) with confirmed EVD received two consecutive transfusions of 200 to 250 ml of ABO-compatible convalescent plasma, with each unit of plasma obtained from a separate convalescent donor. The transfusions were initiated on the day of diagnosis or up to 2 days later. The level of neutralizing antibodies against Ebola virus in the plasma was unknown at the time of administration. The control group was 418 patients who had been treated at the same center during the previous 5 months. The primary outcome was the risk of death during the period from 3 to 16 days after diagnosis with adjustments for age and the baseline cycle-threshold value on polymerase-chain-reaction assay; patients who had died before day 3 were excluded. The clinically important difference was defined as an absolute reduction in mortality of 20 percentage points in the convalescent-plasma group as compared with the control group.
Results: A total of 84 patients who were treated with plasma were included in the primary analysis. At baseline, the convalescent-plasma group had slightly higher cycle-threshold values and a shorter duration of symptoms than did the control group, along with a higher frequency of eye redness and difficulty in swallowing. From day 3 to day 16 after diagnosis, the risk of death was 31% in the convalescent-plasma group and 38% in the control group (risk difference, -7 percentage points; 95% confidence interval [CI], -18 to 4). The difference was reduced after adjustment for age and cycle-threshold value (adjusted risk difference, -3 percentage points; 95% CI, -13 to 8). No serious adverse reactions associated with the use of convalescent plasma were observed.
Conclusions: The transfusion of up to 500 ml of convalescent plasma with unknown levels of neutralizing antibodies in 84 patients with confirmed EVD was not associated with a significant improvement in survival. (Funded by the European Union's Horizon 2020 Research and Innovation Program and others; ClinicalTrials.gov number, NCT02342171.).
Figures
Comment in
-
Convalescent Plasma for Ebola Virus Disease.N Engl J Med. 2016 Jun 23;374(25):2500. doi: 10.1056/NEJMc1602284. N Engl J Med. 2016. PMID: 27332913 No abstract available.
-
Convalescent Plasma for Ebola Virus Disease.N Engl J Med. 2016 Jun 23;374(25):2498-9. doi: 10.1056/NEJMc1602284. N Engl J Med. 2016. PMID: 27332914 No abstract available.
-
Convalescent Plasma for Ebola Virus Disease.N Engl J Med. 2016 Jun 23;374(25):2499. doi: 10.1056/NEJMc1602284. N Engl J Med. 2016. PMID: 27332915 No abstract available.
-
Convalescent Plasma for Ebola Virus Disease.N Engl J Med. 2016 Jun 23;374(25):2499-500. doi: 10.1056/NEJMc1602284. N Engl J Med. 2016. PMID: 27332916 No abstract available.
-
Efficacy of Convalescent Plasma in Relation to Dose of Ebola Virus Antibodies.N Engl J Med. 2016 Dec 8;375(23):2307-2309. doi: 10.1056/NEJMc1609116. Epub 2016 Nov 14. N Engl J Med. 2016. PMID: 27959686 Free PMC article. No abstract available.
-
Convalescent Plasma and the Dose of Ebola Virus Antibodies.N Engl J Med. 2017 Mar 30;376(13):1296-7. doi: 10.1056/NEJMc1700090. N Engl J Med. 2017. PMID: 28355506 No abstract available.
References
-
- World Health Organization. Ebola situation report — September 9, 2015. ( http://apps.who.int/ebola/current-situation/ebola-situation-report-9-sep...)
-
- Bah EI, Lamah MC, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–7. - PubMed
-
- Interim guidance for national health authorities and blood transfusion services: use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. Geneva: World Health Organization; 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
