A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma-borne molecules from the microvasculature
- PMID: 26738537
- PMCID: PMC4807424
- DOI: 10.1182/blood-2015-09-672188
A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma-borne molecules from the microvasculature
Abstract
Previous studies have shown that hemostatic thrombi formed in response to penetrating injuries have a core of densely packed, fibrin-associated platelets overlaid by a shell of less-activated, loosely packed platelets. Here we asked, first, how the diverse elements of this structure combine to stem the loss of plasma-borne molecules and, second, whether antiplatelet agents and anticoagulants that perturb thrombus structure affect the re-establishment of a tight vascular seal. The studies combined high-resolution intravital microscopy with a photo-activatable fluorescent albumin marker to simultaneously track thrombus formation and protein transport following injuries to mouse cremaster muscle venules. The results show that protein loss persists after red cell loss has ceased. Blocking platelet deposition with an αIIbβ3antagonist delays vessel sealing and increases extravascular protein accumulation, as does either inhibiting adenosine 5'-diphosphate (ADP) P2Y12receptors or reducing integrin-dependent signaling and retraction. In contrast, sealing was unaffected by introducing hirudin to block fibrin accumulation or a Gi2α gain-of-function mutation to expand the thrombus shell. Collectively, these observations describe a novel approach for studying vessel sealing after injury in real time in vivo and show that (1) the core/shell architecture previously observed in arterioles also occurs in venules, (2) plasma leakage persists well beyond red cell escape and mature thrombus formation, (3) the most critical events for limiting plasma extravasation are the stable accumulation of platelets, ADP-dependent signaling, and the emergence of a densely packed core, not the accumulation of fibrin, and (4) drugs that affect platelet accumulation and packing can delay vessel sealing, permitting protein escape to continue.
© 2016 by The American Society of Hematology.
Figures

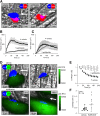



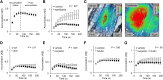

Comment in
-
Platelets stop us leaking.Blood. 2016 Mar 24;127(12):1528-9. doi: 10.1182/blood-2016-01-692186. Blood. 2016. PMID: 27013214
References
-
- Bellido-Martín L, Chen V, Jasuja R, Furie B, Furie BC. Imaging fibrin formation and platelet and endothelial cell activation in vivo. Thromb Haemost. 2011;105(5):776–782. - PubMed
-
- van Gestel MA, Heemskerk JW, Slaaf DW, et al. Real-time detection of activation patterns in individual platelets during thromboembolism in vivo: differences between thrombus growth and embolus formation. J Vasc Res. 2002;39(6):534–543. - PubMed
-
- Hechler B, Nonne C, Eckly A, et al. Arterial thrombosis: relevance of a model with two levels of severity assessed by histologic, ultrastructural and functional characterization. J Thromb Haemost. 2010;8(1):173–184. - PubMed
-
- Hayashi T, Mogami H, Murakami Y, et al. Real-time analysis of platelet aggregation and procoagulant activity during thrombus formation in vivo. Pflugers Arch. 2008;456(6):1239–1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

