A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer
- PMID: 26738797
- PMCID: PMC4794335
- DOI: 10.1126/scitranslmed.aad0293
A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer
Abstract
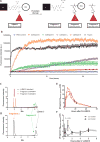
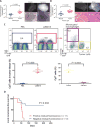
Local recurrence is a common cause of treatment failure for patients with solid tumors. Intraoperative detection of microscopic residual cancer in the tumor bed could be used to decrease the risk of a positive surgical margin, reduce rates of reexcision, and tailor adjuvant therapy. We used a protease-activated fluorescent imaging probe, LUM015, to detect cancer in vivo in a mouse model of soft tissue sarcoma (STS) and ex vivo in a first-in-human phase 1 clinical trial. In mice, intravenous injection of LUM015 labeled tumor cells, and residual fluorescence within the tumor bed predicted local recurrence. In 15 patients with STS or breast cancer, intravenous injection of LUM015 before surgery was well tolerated. Imaging of resected human tissues showed that fluorescence from tumor was significantly higher than fluorescence from normal tissues. LUM015 biodistribution, pharmacokinetic profiles, and metabolism were similar in mouse and human subjects. Tissue concentrations of LUM015 and its metabolites, including fluorescently labeled lysine, demonstrated that LUM015 is selectively distributed to tumors where it is activated by proteases. Experiments in mice with a constitutively active PEGylated fluorescent imaging probe support a model where tumor-selective probe distribution is a determinant of increased fluorescence in cancer. These co-clinical studies suggest that the tumor specificity of protease-activated imaging probes, such as LUM015, is dependent on both biodistribution and enzyme activity. Our first-in-human data support future clinical trials of LUM015 and other protease-sensitive probes.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures




References
-
- Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. - PubMed
-
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. - PubMed
-
- Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. - PubMed
-
- Cendán JC, Coco D, Copeland EM., III Accuracy of intraoperative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg. 2005;201:194–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- 1U43CA165024/CA/NCI NIH HHS/United States
- U43 CA165024/CA/NCI NIH HHS/United States
- R35 CA197743/CA/NCI NIH HHS/United States
- UL1TR001117/TR/NCATS NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- T32GM007171/GM/NIGMS NIH HHS/United States
- T32 GM007171/GM/NIGMS NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- 5P30-CA-014236-38/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

