Seasonal Effectiveness of Live Attenuated and Inactivated Influenza Vaccine
- PMID: 26738884
- PMCID: PMC4732363
- DOI: 10.1542/peds.2015-3279
Seasonal Effectiveness of Live Attenuated and Inactivated Influenza Vaccine
Abstract
Background: Few observational studies have evaluated the relative effectiveness of live attenuated (LAIV) and inactivated (IIV) influenza vaccines against medically attended laboratory-confirmed influenza.
Methods: We analyzed US Influenza Vaccine Effectiveness Network data from participants aged 2 to 17 years during 4 seasons (2010-2011 through 2013-2014) to compare relative effectiveness of LAIV and IIV against influenza-associated illness. Vaccine receipt was confirmed via provider/electronic medical records or immunization registry. We calculated the ratio (odds) of influenza-positive to influenza-negative participants among those age-appropriately vaccinated with either LAIV or IIV for the corresponding season. We examined relative effectiveness of LAIV and IIV by using adjusted odds ratios (ORs) and 95% confidence intervals (CIs) from logistic regression.
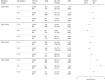
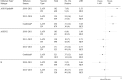
Results: Of 6819 participants aged 2 to 17 years, 2703 were age-appropriately vaccinated with LAIV (n = 637) or IIV (n = 2066). Odds of influenza were similar for LAIV and IIV recipients during 3 seasons (2010-2011 through 2012-2013). In 2013-2014, odds of influenza were significantly higher among LAIV recipients compared with IIV recipients 2 to 8 years old (OR 5.36; 95% CI, 2.37 to 12.13). Participants vaccinated with LAIV or IIV had similar odds of illness associated with influenza A/H3N2 or B. LAIV recipients had greater odds of illness due to influenza A/H1N1pdm09 in 2010-2011 and 2013-2014.
Conclusions: We observed lower effectiveness of LAIV compared with IIV against influenza A/H1N1pdm09 but not A(H3N2) or B among children and adolescents, suggesting poor performance related to the LAIV A/H1N1pdm09 viral construct.
Copyright © 2016 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
References
-
- Fiore AE, Shay DK, Broder K, et al. ; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) . Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60 - PubMed
-
- Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001;20(8):733–740 - PubMed
-
- Belshe RB, Edwards KM, Vesikari T, et al. ; CAIV-T Comparative Efficacy Study Group . Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–696 - PubMed
-
- Fleming DM, Crovari P, Wahn U, et al. ; CAIV-T Asthma Study Group . Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25(10):860–869 - PubMed
-
- Ashkenazi S, Vertruyen A, Arístegui J, et al. ; CAIV-T Study Group . Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25(10):870–879 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR024153/RR/NCRR NIH HHS/United States
- U01 IP000172/IP/NCIRD CDC HHS/United States
- U18 IP000172/IP/NCIRD CDC HHS/United States
- U01 IP000170/IP/NCIRD CDC HHS/United States
- UL1TR000005/TR/NCATS NIH HHS/United States
- U01 IP000183/IP/NCIRD CDC HHS/United States
- U18 IP000184/IP/NCIRD CDC HHS/United States
- U18 IP000183/IP/NCIRD CDC HHS/United States
- U01 IP000473/IP/NCIRD CDC HHS/United States
- U01 IP000466/IP/NCIRD CDC HHS/United States
- U01 IP000184/IP/NCIRD CDC HHS/United States
- U01IP000474/IP/NCIRD CDC HHS/United States
- U01 IP000471/IP/NCIRD CDC HHS/United States
- U01 IP001037/IP/NCIRD CDC HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U01IP000467/IP/NCIRD CDC HHS/United States
- CC999999/ImCDC/Intramural CDC HHS/United States
- U01 IP000474/IP/NCIRD CDC HHS/United States
- U01 IP000467/IP/NCIRD CDC HHS/United States
- U18 IP000170/IP/NCIRD CDC HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

