Label-free cell cycle analysis for high-throughput imaging flow cytometry
- PMID: 26739115
- PMCID: PMC4729834
- DOI: 10.1038/ncomms10256
Label-free cell cycle analysis for high-throughput imaging flow cytometry
Abstract
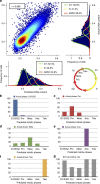
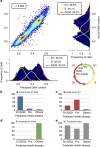
Imaging flow cytometry combines the high-throughput capabilities of conventional flow cytometry with single-cell imaging. Here we demonstrate label-free prediction of DNA content and quantification of the mitotic cell cycle phases by applying supervised machine learning to morphological features extracted from brightfield and the typically ignored darkfield images of cells from an imaging flow cytometer. This method facilitates non-destructive monitoring of cells avoiding potentially confounding effects of fluorescent stains while maximizing available fluorescence channels. The method is effective in cell cycle analysis for mammalian cells, both fixed and live, and accurately assesses the impact of a cell cycle mitotic phase blocking agent. As the same method is effective in predicting the DNA content of fission yeast, it is likely to have a broad application to other cell types.
Conflict of interest statement
Although the software described is completely open-source, a provisional patent application has been filed relating to the method proposed in this manuscript.
Figures



References
-
- Brown M. & Wittwer C. Flow cytometry: principles and clinical applications in hematology. Clin. Chem. 46, 1221–1229 (2000) . - PubMed
-
- Darzynkiewicz Z. & Huang X. Analysis of cellular DNA content by flow cytometry. Curr. Protoc. Immunol. 5, 7 (2004) . - PubMed
-
- Hans F. & Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene 20, 3021–3027 (2001) . - PubMed
-
- Sakaue-Sawano A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

