Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress
- PMID: 26739489
- PMCID: PMC4796279
- DOI: 10.1152/ajpcell.00171.2015
Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress
Abstract
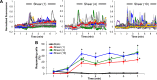
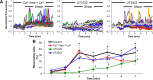
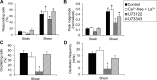
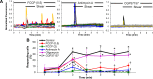
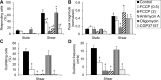
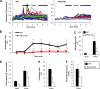
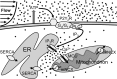
Shear stress is known to stimulate an intracellular free calcium concentration ([Ca(2+)]i) response in vascular endothelial cells (ECs). [Ca(2+)]i is a key second messenger for signaling that leads to vasodilation and EC survival. Although it is accepted that the shear-induced [Ca(2+)]i response is, in part, due to Ca(2+) release from the endoplasmic reticulum (ER), the role of mitochondria (second largest Ca(2+) store) is unknown. We hypothesized that the mitochondria play a role in regulating [Ca(2+)]i in sheared ECs. Cultured ECs, loaded with a Ca(2+)-sensitive fluorophore, were exposed to physiological levels of shear stress. Shear stress elicited [Ca(2+)]i transients in a percentage of cells with a fraction of them displaying oscillations. Peak magnitudes, percentage of oscillating ECs, and oscillation frequencies depended on the shear level. [Ca(2+)]i transients/oscillations were present when experiments were conducted in Ca(2+)-free solution (plus lanthanum) but absent when ECs were treated with a phospholipase C inhibitor, suggesting that the ER inositol 1,4,5-trisphosphate receptor is responsible for the [Ca(2+)]i response. Either a mitochondrial uncoupler or an electron transport chain inhibitor, but not a mitochondrial ATP synthase inhibitor, prevented the occurrence of transients and especially inhibited the oscillations. Knockdown of the mitochondrial Ca(2+) uniporter also inhibited the shear-induced [Ca(2+)]i transients/oscillations compared with controls. Hence, EC mitochondria, through Ca(2+) uptake/release, regulate the temporal profile of shear-induced ER Ca(2+) release. [Ca(2+)]i oscillation frequencies detected were within the range for activation of mechanoresponsive kinases and transcription factors, suggesting that dysfunctional EC mitochondria may contribute to cardiovascular disease by deregulating the shear-induced [Ca(2+)]i response.
Keywords: calcium oscillations; endothelial cell; fluid shear stress; intracellular calcium; mitochondria.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Ando J, Yamamoto K. Flow detection and calcium signaling in vascular endothelial cells. Cardiovasc Res 99: 260–268, 2013. - PubMed
-
- Arnaudeau S, Kelley WL, Walsh JV Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem 276: 29430–29439, 2001. - PubMed
-
- Belmonte S, Morad M. Shear fluid-induced Ca2+ release and the role of mitochondria in rat cardiac myocytes. Ann NY Acad Sci 1123: 58–63, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

