Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study
- PMID: 26739731
- PMCID: PMC4716252
- DOI: 10.1136/bmjopen-2015-009536
Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study
Abstract
Objective: Pertussis vaccination during pregnancy has recently been recommended in both the USA and UK to prevent pertussis infection in infants. While there are no apparent safety concerns about the administration of Tdap vaccine during pregnancy, there is only limited safety data available. We aimed to closely monitor infants exposed to Tdap during pregnancy to look for any adverse outcomes that may be attributable to the vaccine.
Design: This was a prospective observational study, collecting information to evaluate the safety of Tdap vaccine for infants exposed during pregnancy. Infants were followed for between 6 and 12 months after birth, with 84% completing 12 months of follow-up. Information was obtained from objective sources including routine health visits and vaccination records wherever possible, as well as frequent parental reports.
Setting: The Canterbury region of New Zealand.
Patients: A cohort of 403 infants whose mothers had received Tdap vaccine.
Main outcome measures: Gestational age at birth, growth parameters, congenital anomalies, immunisation status and timeliness of immunisation, development of pertussis infection.
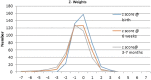
Results: There were no significant differences in birth weight, gestational age at birth, congenital anomalies or infant growth as compared with baseline population data. Infants of mothers who had received the vaccine were more likely to receive their vaccinations on time during infancy. No cases of pertussis occurred in this cohort despite high rates of disease in the community. We have not found any adverse events attributable to vaccine exposure.
Conclusions: These data add to the growing pool of evidence that the administration of Tdap vaccine during pregnancy is an appropriate strategy for reducing the burden of pertussis in infants.
Clinical trial registration: Australia New Zealand Clinical Trials Registry ACTRN12613001045707.
Keywords: INFECTIOUS DISEASES; PUBLIC HEALTH.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
-
- https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-... JCVI meeting on pertussis immunisation: August 2012.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical