Evidence for new C-terminally truncated variants of α- and β-tubulins
- PMID: 26739754
- PMCID: PMC4750924
- DOI: 10.1091/mbc.E15-03-0137
Evidence for new C-terminally truncated variants of α- and β-tubulins
Abstract
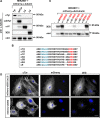
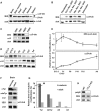
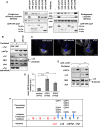
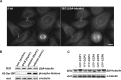
Cellular α-tubulin can bear various carboxy-terminal sequences: full-length tubulin arising from gene neosynthesis is tyrosinated, and two truncated variants, corresponding to detyrosinated and Δ2 α‑tubulin, result from the sequential cleavage of one or two C-terminal residues, respectively. Here, by using a novel antibody named 3EG that is highly specific to the -EEEG C-terminal sequence, we demonstrate the occurrence in neuronal tissues of a new αΔ3‑tubulin variant corresponding to α1A/B‑tubulin deleted of its last three residues (EEY). αΔ3‑tubulin has a specific distribution pattern: its quantity in the brain is similar to that of αΔ2-tubulin around birth but is much lower in adult tissue. This truncated α1A/B-tubulin variant can be generated from αΔ2-tubulin by the deglutamylases CCP1, CCP4, CCP5, and CCP6 but not by CCP2 and CCP3. Moreover, using 3EG antibody, we identify a C‑terminally truncated β-tubulin form with the same -EEEG C-terminal sequence. Using mass spectrometry, we demonstrate that β2A/B-tubulin is modified by truncation of the four C-terminal residues (EDEA). We show that this newly identified βΔ4-tubulin is ubiquitously present in cells and tissues and that its level is constant throughout the cell cycle. These new C-terminally truncated α- and β-tubulin variants, both ending with -EEEG sequence, are expected to regulate microtubule physiology. Of interest, the αΔ3-tubulin seems to be related to dynamic microtubules, resembling tyrosinated-tubulin rather than the other truncated variants, and may have critical function(s) in neuronal development.
© 2016 Aillaud et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

