α-Synuclein interferes with the ESCRT-III complex contributing to the pathogenesis of Lewy body disease
- PMID: 26740557
- PMCID: PMC4764192
- DOI: 10.1093/hmg/ddv633
α-Synuclein interferes with the ESCRT-III complex contributing to the pathogenesis of Lewy body disease
Abstract
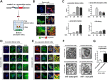
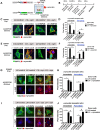
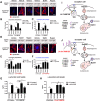
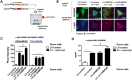
α-Synuclein (α-syn) has been implicated in neurological disorders with parkinsonism, including Parkinson's disease and Dementia with Lewy body. Recent studies have shown α-syn oligomers released from neurons can propagate from cell-to-cell in a prion-like fashion exacerbating neurodegeneration. In this study, we examined the role of the endosomal sorting complex required for transport (ESCRT) pathway on the propagation of α-syn. α-syn, which is transported via the ESCRT pathway through multivesicular bodies for degradation, can also target the degradation of the ESCRT protein-charged multivesicular body protein (CHMP2B), thus generating a roadblock of endocytosed α-syn. Disruption of the ESCRT transport system also resulted in increased exocytosis of α-syn thus potentially increasing cell-to-cell propagation of synuclein. Conversely, delivery of a lentiviral vector overexpressing CHMP2B rescued the neurodegeneration in α-syn transgenic mice. Better understanding of the mechanisms of intracellular trafficking of α-syn might be important for understanding the pathogenesis and developing new treatments for synucleinopathies.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
ESCRT-mediated uptake and degradation of brain-targeted α-synuclein single chain antibody attenuates neuronal degeneration in vivo.Mol Ther. 2014 Oct;22(10):1753-1767. doi: 10.1038/mt.2014.129. Epub 2014 Jul 10. Mol Ther. 2014. Retraction in: Mol Ther. 2025 Mar 5;33(3):1302. doi: 10.1016/j.ymthe.2025.01.027. PMID: 25008355 Free PMC article. Retracted.
-
Immunotherapy targeting toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating α-synuclein transmission and neuroinflammation.Mol Neurodegener. 2018 Aug 9;13(1):43. doi: 10.1186/s13024-018-0276-2. Mol Neurodegener. 2018. PMID: 30092810 Free PMC article.
-
14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic α-Synuclein.J Neurosci. 2018 Sep 19;38(38):8211-8232. doi: 10.1523/JNEUROSCI.1134-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093536 Free PMC article.
-
Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
-
Formation and development of Lewy pathology: a critical update.J Neurol. 2009 Aug;256 Suppl 3:270-9. doi: 10.1007/s00415-009-5243-y. J Neurol. 2009. PMID: 19711116 Review.
Cited by
-
Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer's Disease.Front Cell Neurosci. 2018 Sep 24;12:317. doi: 10.3389/fncel.2018.00317. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30319358 Free PMC article. Review.
-
The yeast guanine nucleotide exchange factor Sec7 is a bottleneck in spatial protein quality control and detoxifies neurological disease proteins.Sci Rep. 2023 Aug 28;13(1):14068. doi: 10.1038/s41598-023-41188-0. Sci Rep. 2023. PMID: 37640758 Free PMC article.
-
Dysregulation of Rab5-mediated endocytic pathways in Alzheimer's disease.Traffic. 2018 Apr;19(4):253-262. doi: 10.1111/tra.12547. Epub 2018 Feb 5. Traffic. 2018. PMID: 29314494 Free PMC article. Review.
-
Systemic peptide mediated delivery of an siRNA targeting α-syn in the CNS ameliorates the neurodegenerative process in a transgenic model of Lewy body disease.Neurobiol Dis. 2019 Jul;127:163-177. doi: 10.1016/j.nbd.2019.03.001. Epub 2019 Mar 5. Neurobiol Dis. 2019. PMID: 30849508 Free PMC article.
-
Acute Transcriptomic and Epigenetic Alterations at T12 After Rat T10 Spinal Cord Contusive Injury.Mol Neurobiol. 2023 May;60(5):2937-2953. doi: 10.1007/s12035-023-03250-w. Epub 2023 Feb 8. Mol Neurobiol. 2023. PMID: 36750527
References
-
- Nakamura T., Lipton S.A. (2009) Cell death: protein misfolding and neurodegenerative diseases. Apoptosis, 14, 455–468. - PubMed
-
- Martin F.L., Williamson S.J., Paleologou K.E., Allsop D., El-Agnaf O.M. (2004) Alpha-synuclein and the pathogenesis of Parkinson's disease. Protein Pept. Lett., 11, 229–237. - PubMed
-
- Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. (1997) Alpha-synuclein in Lewy bodies. Nature, 388, 839–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

