Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells
- PMID: 26740577
- PMCID: PMC4716810
- DOI: 10.1093/humrep/dev320
Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells
Abstract
Study question: Which receptors for prostaglandin E2 (PGE2) and vascular endothelial growth factor A (VEGFA) mediate angiogenesis in the human follicle around the time of ovulation?
Summary answer: PGE2 and VEGFA act via multiple PGE2 receptors (PTGERs) and VEGF receptors (VEGFRs) to play complementary roles in follicular angiogenesis.
What is known already: Production of PGE2 and VEGFA by the follicle are prerequisites for ovulation. PGE2 is an emerging regulator of angiogenesis and has not been examined in the context of the human ovulatory follicle. VEGFA is an established regulator of follicular angiogenesis.
Study design, size, duration: Ovarian biopsies containing the ovulatory follicle were obtained from 11 women of reproductive age (30-45 years) undergoing surgery for laparoscopic sterilization. In some cases, women received hCG to substitute for the ovulatory LH surge before ovarian biopsy. In addition, aspirates from four women of reproductive age (18-31 years) undergoing gonadotrophin stimulation for oocyte donation were obtained for isolation of human ovarian microvascular endothelial cells (hOMECs).
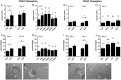
Participants/materials, setting, methods: Ovarian biopsies were utilized for immunocytochemical detection of von Willebrand factor to identify endothelial cells. hOMECs were cultured with PGE2, PTGER receptor selective agonists, VEGFA, or VEGFR selective agonists. hOMECs were assessed for proliferation by Ki67 immunocytochemistry. hOMEC migration was determined by counting cells which migrated through a porous membrane in vitro. Sprout formation was quantified by determining sprout number and length from photographs take after culture of hOMECs in a 3-dimensional matrix.
Main results and the role of chance: Endothelial cells were not observed within the granulosa cell layer of human ovulatory follicles prior to an ovulatory dose of hCG and were first seen amongst granulosa cells 18-34 h after hCG. In vitro, PGE2 enhanced migration and sprout formation but did not alter hOMEC proliferation. Agonists selective for each PTGER increased migration with no change in proliferation. PTGER1 and PTGER2 agonists increased the number of sprouts, while only PTGER1 affected sprout length. VEGFA increased hOMEC proliferation, migration, and formation of structures resembling capillary sprouts. Signaling through VEGFR1 promoted hOMEC migration, proliferation, and the formation of few, long endothelial cell sprouts, while VEGFR2 stimulation promoted hOMEC migration and the formation of many, short sprouts. All effects of treatments in vitro were considered significant at P < 0.05.
Limitations, reasons for caution: While primary cultures of hOMECs respond to PGE2 and VEGFA differently than other cultured endothelial cells, hOMECs may not respond to PGE2 and VEGFA in vivo as they do in vitro.
Wider implications of the findings: Agonists and antagonists selective for PTGER1, PTGER2, VEGFR1, or VEGFR2 may have therapeutic value to promote or prevent ovulation in women.
Study funding/competing interests: This research was supported by grant funding from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (HD071875 to D.M.D., T.E.C., M.B.). The authors have no conflicts of interest to disclose.
Keywords: PGE2 receptor; VEGF receptor; endothelial cell; follicle; ovary; ovulation; prostaglandin; vascular endothelial growth factor.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


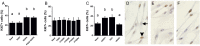
Similar articles
-
Luteinizing hormone receptor promotes angiogenesis in ovarian endothelial cells of Macaca fascicularis and Homo sapiens†.Biol Reprod. 2023 Feb 13;108(2):258-268. doi: 10.1093/biolre/ioac189. Biol Reprod. 2023. PMID: 36214501 Free PMC article.
-
Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2.Biol Reprod. 2015 Jan;92(1):15. doi: 10.1095/biolreprod.114.123711. Epub 2014 Nov 5. Biol Reprod. 2015. PMID: 25376231 Free PMC article.
-
Vascular endothelial growth factors C and D may promote angiogenesis in the primate ovulatory follicle.Biol Reprod. 2017 Feb 1;96(2):389-400. doi: 10.1095/biolreprod.116.144733. Biol Reprod. 2017. PMID: 28203718 Free PMC article.
-
Mapping PTGERs to the Ovulatory Follicle: Regional Responses to the Ovulatory PGE2 Signal.Biol Reprod. 2016 Aug;95(2):33. doi: 10.1095/biolreprod.116.140574. Epub 2016 Jun 15. Biol Reprod. 2016. PMID: 27307073 Free PMC article. Review.
-
Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway.Hum Reprod Update. 2015 Sep-Oct;21(5):652-70. doi: 10.1093/humupd/dmv026. Epub 2015 May 29. Hum Reprod Update. 2015. PMID: 26025453 Free PMC article. Review.
Cited by
-
Luteinizing hormone receptor promotes angiogenesis in ovarian endothelial cells of Macaca fascicularis and Homo sapiens†.Biol Reprod. 2023 Feb 13;108(2):258-268. doi: 10.1093/biolre/ioac189. Biol Reprod. 2023. PMID: 36214501 Free PMC article.
-
Targeting EP2 receptor with multifaceted mechanisms for high-risk neuroblastoma.Cell Rep. 2022 Jun 21;39(12):111000. doi: 10.1016/j.celrep.2022.111000. Cell Rep. 2022. PMID: 35732130 Free PMC article.
-
Neurotensin: A novel mediator of ovulation?FASEB J. 2021 Apr;35(4):e21481. doi: 10.1096/fj.202002547RR. FASEB J. 2021. PMID: 33710668 Free PMC article.
-
Prostaglandins in Superovulation Induced Bovine Follicles During the Preovulatory Period and Early Corpus Luteum.Front Endocrinol (Lausanne). 2019 Jul 10;10:467. doi: 10.3389/fendo.2019.00467. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31354631 Free PMC article.
-
The Unique Mechanisms of Cellular Proliferation, Migration and Apoptosis are Regulated through Oocyte Maturational Development-A Complete Transcriptomic and Histochemical Study.Int J Mol Sci. 2018 Dec 26;20(1):84. doi: 10.3390/ijms20010084. Int J Mol Sci. 2018. PMID: 30587792 Free PMC article.
References
-
- Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci 2004;82-83:127–140. - PubMed
-
- Arslan M, Bocca S, Mirkin S, Barroso G, Stadtmauer L, Oehninger S. Controlled ovarian hyperstimulation protocols for in vitro fertilization: two decades of experience after the birth of Elizabeth Carr. Fertil Steril 2005;84:555–569. - PubMed
-
- Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem 1999;274:31047–31054. - PubMed
-
- Bohgaki M, Kitaguchi H. Conversion of cultured monocytes/macrophages into endothelial-like cells through direct contact with endothelial cells. Int J Hematol 2007;86:42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

