Use of Cysteine Trapping to Map Spatial Approximations between Residues Contributing to the Helix N-capping Motif of Secretin and Distinct Residues within Each of the Extracellular Loops of Its Receptor
- PMID: 26740626
- PMCID: PMC4777851
- DOI: 10.1074/jbc.M115.706010
Use of Cysteine Trapping to Map Spatial Approximations between Residues Contributing to the Helix N-capping Motif of Secretin and Distinct Residues within Each of the Extracellular Loops of Its Receptor
Abstract
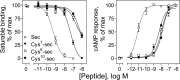
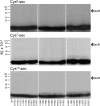
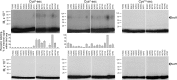
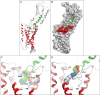
Amino-terminal regions of secretin-family peptides contain key determinants for biological activity and binding specificity, although the nature of interactions with receptors is unclear. A helix N-capping motif within this region has been postulated to directly contribute to agonist activity while also stabilizing formation of a helix extending toward the peptide carboxyl terminus and docking within the receptor amino terminus. We used cysteine trapping to systematically explore spatial approximations between cysteines replacing each residue in this motif of secretin (sec), Phe(6), Thr(7), and Leu(10), and cysteines incorporated into the extracellular face of the receptor. Each peptide was a full agonist for cAMP, but had a lower binding affinity than natural hormone. These bound to COS cells expressing 61 receptor constructs incorporating cysteines in every position along each extracellular loop (ECL) and adjacent parts of transmembrane (TM) segments. Patterns of covalent labeling were distinct for each probe, with Cys(6)-sec labeling multiple residues in the carboxyl-terminal half of ECL2 and throughout ECL3, Cys(7)-sec predominantly labeling only single residues in the carboxyl-terminal end of ECL2 and the amino-terminal end of ECL3, and Cys(10)-sec not efficiently labeling any of these residues. These spatial constraints were used to refine our model of secretin bound to its receptor, now bringing ECL3 above the amino terminus of the ligand and revealing possible charge-charge interactions between this part of secretin and receptor residues in TM5, TM6, ECL2, and ECL3, which can orient and stabilize the peptide-receptor complex. This was validated by testing predicted approximations by mutagenesis and residue-residue complementation studies.
Keywords: G protein-coupled receptor (GPCR); cell surface receptor; cyclic AMP (cAMP); gel electrophoresis; ligand-binding protein; membrane protein; molecular modeling; mutagenesis; peptides.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
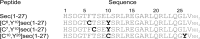




References
-
- Neumann J. M., Couvineau A., Murail S., Lacapère J. J., Jamin N., and Laburthe M. (2008) Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem. Sci. 33, 314–319 - PubMed
-
- Miller L. J., Dong M., Harikumar K. G., and Gao F. (2007) Structural basis of natural ligand binding and activation of the Class II G-protein-coupled secretin receptor. Biochem. Soc. Trans. 35, 709–712 - PubMed
-
- Miller L. J., and Dong M. (2013) The orthosteric agonist-binding pocket in the prototypic class B G-protein-coupled secretin receptor. Biochem. Soc. Trans. 41, 154–158 - PubMed
-
- Ulrich C. D. 2nd, Holtmann M., and Miller L. J. (1998) Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology 114, 382–397 - PubMed
-
- Dong M., Xu X., Ball A. M., Makhoul J. A., Lam P. C., Pinon D. I., Orry A., Sexton P. M., Abagyan R., and Miller L. J. (2012) Mapping spatial approximations between the amino terminus of secretin and each of the extracellular loops of its receptor using cysteine trapping. FASEB J. 26, 5092–5105 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

