Spontaneous Pancreatitis Caused by Tissue-Specific Gene Ablation of Hhex in Mice
- PMID: 26740970
- PMCID: PMC4698881
- DOI: 10.1016/j.jcmgh.2015.06.007
Spontaneous Pancreatitis Caused by Tissue-Specific Gene Ablation of Hhex in Mice
Abstract
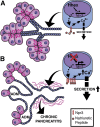
Background & aims: Perturbations in pancreatic ductal bicarbonate secretion cause chronic pancreatitis. The physiologic mechanism of ductal secretion is known, but its transcriptional control is not. We determine the role of the transcription factor hematopoietically expressed homeobox protein (Hhex) in ductal secretion and pancreatitis.
Methods: We derived mice with pancreas-specific, Cremediated Hhex gene ablation to determine the requirement of Hhex in the pancreatic duct in early life and in adult stages. Histologic and immunostaining analyses were used to detect the presence of pathology. Pancreatic primary ductal cells were isolated to discover differentially expressed transcripts upon acute Hhex ablation on a cell autonomous level.
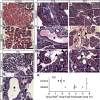
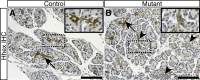
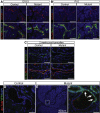
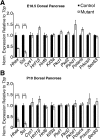
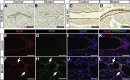
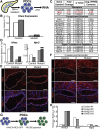
Results: Hhex protein was detected throughout the embryonic and adult ductal trees. Ablation of Hhex in pancreatic progenitors resulted in postnatal ductal ectasia associated with acinar-to-ductal metaplasia, a progressive phenotype that ultimately resulted in chronic pancreatitis. Hhex ablation in adult mice, however, did not cause any detectable pathology. Ductal ectasia in young mice did not result from perturbation of expression of Hnf6, Hnf1β, or the primary cilia genes. RNA-seq analysis of Hhex-ablated pancreatic primary ductal cells showed mRNA levels of the G-protein coupled receptor natriuretic peptide receptor 3 (Npr3), implicated in paracrine signaling, up-regulated by 4.70-fold.
Conclusions: Although Hhex is dispensable for ductal cell function in the adult, ablation of Hhex in pancreatic progenitors results in pancreatitis. Our data highlight the critical role of Hhex in maintaining ductal homeostasis in early life and support ductal hypersecretion as a novel etiology of pediatric chronic pancreatitis.
Keywords: Npr3; Pancreatic Ducts; Primary Cilia.
Figures





References
-
- Whitcomb D.C., Ermentrout G.B. A mathematical model of the pancreatic duct cell generating high bicarbonate concentrations in pancreatic juice. Pancreas. 2004;29:e30–e40. - PubMed
-
- Folsch U.R., Fischer H., Soling H.D. Effects of gastrointestinal hormones and carbamylcholine on cAMP accumulation in isolated pancreatic duct fragments from the rat. Digestion. 1980;20:277–292. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
