Stable Colloidal Drug Aggregates Catch and Release Active Enzymes
- PMID: 26741163
- PMCID: PMC5082698
- DOI: 10.1021/acschembio.5b00806
Stable Colloidal Drug Aggregates Catch and Release Active Enzymes
Abstract
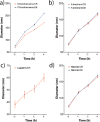
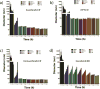
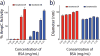
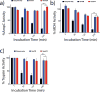
Small molecule aggregates are considered nuisance compounds in drug discovery, but their unusual properties as colloids could be exploited to form stable vehicles to preserve protein activity. We investigated the coaggregation of seven molecules chosen because they had been previously intensely studied as colloidal aggregators, coformulating them with bis-azo dyes. The coformulation reduced colloid sizes to <100 nm and improved uniformity of the particle size distribution. The new colloid formulations are more stable than previous aggregator particles. Specifically, coaggregation of Congo Red with sorafenib, tetraiodophenolphthalein (TIPT), or vemurafenib produced particles that are stable in solutions of high ionic strength and high protein concentrations. Like traditional, single compound colloidal aggregates, the stabilized colloids adsorbed and inhibited enzymes like β-lactamase, malate dehydrogenase, and trypsin. Unlike traditional aggregates, the coformulated colloid-protein particles could be centrifuged and resuspended multiple times, and from resuspended particles, active trypsin could be released up to 72 h after adsorption. Unexpectedly, the stable colloidal formulations can sequester, stabilize, and isolate enzymes by spin-down, resuspension, and release.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures



References
-
- Brick MC, Palmer HJ, Whitesides TH. Formation of colloidal dispersions of organic materials in aqueous media by solvent shifting. Langmuir. 2003;19:6367–6380.
-
- McGovern SL, Caselli E, Grigorieff N, Shoichet BK. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J Med Chem. 2002;45:1712–1722. - PubMed
-
- McGovern SL, Helfand BT, Feng B, Shoichet BK. A specific mechanism of nonspecific inhibition. J Med Chem. 2003;46:4265–4272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

