An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia
- PMID: 26741285
- PMCID: PMC4908650
- DOI: 10.1038/npp.2016.2
An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia
Abstract
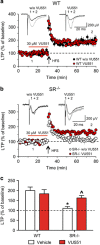
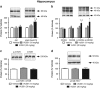
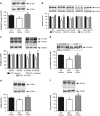
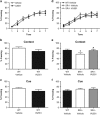
There is substantial evidence that NMDA receptor (NMDAR) hypofunction contributes to the pathophysiology of schizophrenia (SCZ). A recent large-scale genome-wide association study identified serine racemase (SR), the enzyme that produces the NMDAR co-agonist D-serine, as a risk gene for SCZ. Serine racemase knockout (SR-/-) mice, which lack D-serine, exhibit many of the neurochemical and behavioral abnormalities observed in SCZ. Metabotropic glutamate receptor 5 (mGlu5)-positive allosteric modulators (PAMs) are currently being developed to treat cognitive dysfunction. We used in vitro electrophysiology to determine whether the mGlu5 PAM VU0409551 directly enhances NMDAR function in hippocampal slices from adult male SR-/- mice. We administered VU0409551 systemically for 5 days to adult male wild-type C57BL/6 animals to determine the optimal dose to test in SR-/- mice. We used western blot analyses and trace-fear conditioning to determine whether 5 days of VU0409551 treatment could reverse the neuroplasticity and learning deficits, respectively, in SR-/- mice. We show that VU0409551 enhances NMDAR function and rescues long-term potentiation in hippocampal slices obtained from SR-/- mice. Systemic treatment with VU0409551 (10 and 30 mg/kg) to wild-type mice causes a dose-dependent increase in the Akt/GS3Kα/β signaling pathway, which is reduced in SR-/- mice and in SCZ. Furthermore, the administration of VU0409551 to SR-/- mice reverses their deficits in several neuroplasticity signaling pathways and improves their contextual fear memory. These results support positive allosteric modulation of mGlu5, particularly with VU0409551, as a viable mechanism to reverse the deficits in NMDAR function, synaptic plasticity, and memory that are known to be impaired in SCZ.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

