Regulator of cullins-1 expression knockdown suppresses the malignant progression of muscle-invasive transitional cell carcinoma by regulating mTOR/DEPTOR pathway
- PMID: 26742010
- PMCID: PMC4742580
- DOI: 10.1038/bjc.2015.444
Regulator of cullins-1 expression knockdown suppresses the malignant progression of muscle-invasive transitional cell carcinoma by regulating mTOR/DEPTOR pathway
Abstract
Background: Regulator of cullins-1 (ROC1) is a key subunit in the cullin-RING ligase (CRL) protein complex. Our previous study indicated that ROC1 was essential for bladder cancer cell survival and that ROC1 knockdown inhibited CRL activity, triggering G2 phase arrest and senescence. However, the role of ROC1 in the malignant progression of bladder cancer remained unknown.
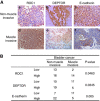
Methods: ROC1 expression in cancer cells was knocked down by siRNA silencing. The effects of ROC1 silencing were evaluated by in vitro assays for cell migration and by an in vivo mouse metastasis model. Epithelial-mesenchymal transition (EMT) induction was evaluated by immunofluorescence staining and western blotting of EMT-associated proteins. ROC1 expression in human tumours was further evaluated by immunohistochemical analysis.
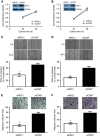
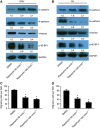
Results: ROC1 knockdown suppresses bladder cancer cell migration by inhibiting EMT. ROC1 knockdown inhibited EMT by inhibiting mammalian target of rapamycin (mTOR) activity via the accumulation of the mTOR-inhibitory protein DEPTOR, a CRL substrate. DEPTOR knockdown partially rescued ROC1 knockdown-inhibited EMT and the ROC1-induced inhibition of cancer cell migration. Furthermore, in vivo studies using a nude mouse metastasis model confirmed the in vitro data. Finally, tissue microarray analysis of clinical bladder cancer specimens indicated a positive correlation between ROC1 expression and EMT.
Conclusions: ROC1 has an important role in the malignant progression of bladder cancer via the mTOR/DEPTOR pathway. ROC1 may serve as a novel therapeutic target for the treatment of muscle-invasive transitional cell carcinoma.
Figures



Similar articles
-
Knockdown of regulator of cullins-1 (ROC1) expression induces bladder cancer cell cycle arrest at the G2 phase and senescence.PLoS One. 2013 May 8;8(5):e62734. doi: 10.1371/journal.pone.0062734. Print 2013. PLoS One. 2013. PMID: 23667514 Free PMC article.
-
Down-regulating ribonuclease inhibitor enhances metastasis of bladder cancer cells through regulating epithelial-mesenchymal transition and ILK signaling pathway.Exp Mol Pathol. 2014 Jun;96(3):411-21. doi: 10.1016/j.yexmp.2014.04.012. Epub 2014 Apr 24. Exp Mol Pathol. 2014. PMID: 24768914
-
Induction of autophagy and senescence by knockdown of ROC1 E3 ubiquitin ligase to suppress the growth of liver cancer cells.Cell Death Differ. 2013 Feb;20(2):235-47. doi: 10.1038/cdd.2012.113. Epub 2012 Aug 31. Cell Death Differ. 2013. PMID: 22935614 Free PMC article.
-
Targeting the mTOR-DEPTOR pathway by CRL E3 ubiquitin ligases: therapeutic application.Neoplasia. 2012 May;14(5):360-7. doi: 10.1593/neo.12532. Neoplasia. 2012. PMID: 22745582 Free PMC article. Review.
-
The role of Ras superfamily proteins in bladder cancer progression.J Urol. 2003 Nov;170(5):1987-93. doi: 10.1097/01.ju.0000088670.02905.78. J Urol. 2003. PMID: 14532839 Review.
Cited by
-
Regulator of cullins-1 (ROC1) negatively regulates the Gli2 regulator SUFU to activate the hedgehog pathway in bladder cancer.Cancer Cell Int. 2021 Jan 26;21(1):75. doi: 10.1186/s12935-021-01775-5. Cancer Cell Int. 2021. PMID: 33499884 Free PMC article.
-
ROC-1, P21 and CAIX as markers of tumor aggressiveness in bladder carcinoma in Egyptian patients.Diagn Pathol. 2020 Apr 7;15(1):33. doi: 10.1186/s13000-020-00947-7. Diagn Pathol. 2020. PMID: 32264924 Free PMC article.
-
ROC1 promotes the malignant progression of bladder cancer by regulating p-IκBα/NF-κB signaling.J Exp Clin Cancer Res. 2021 May 7;40(1):158. doi: 10.1186/s13046-021-01935-5. J Exp Clin Cancer Res. 2021. PMID: 33962660 Free PMC article.
-
E3 ubiquitin ligases and deubiquitinases in bladder cancer tumorigenesis and implications for immunotherapies.Front Immunol. 2023 Jul 11;14:1226057. doi: 10.3389/fimmu.2023.1226057. eCollection 2023. Front Immunol. 2023. PMID: 37497216 Free PMC article. Review.
-
MiR-378 and MiR-1827 Regulate Tumor Invasion, Migration and Angiogenesis in Human Lung Adenocarcinoma by Targeting RBX1 and CRKL, Respectively.J Cancer. 2018 Jan 1;9(2):331-345. doi: 10.7150/jca.18188. eCollection 2018. J Cancer. 2018. PMID: 29344280 Free PMC article.
References
-
- Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M, Wang XA, Zhang F, Jiang L, Zhang Y, Hu Y, Xiang S, Shu Y, Bao R, Li H, Wu W, Weng H, Yen Y, Liu Y (2015) Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett 360(2): 141–150. - PubMed
-
- Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, Lyssiotis CA, Gygi SP, Toker A, Cantley LC, Asara JM, Harper JW, Wei W (2011) mTOR drives its own activation via SCF(betaTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell 44(2): 290–303. - PMC - PubMed
-
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12(2): 89–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

