Regulator of cullins-1 expression knockdown suppresses the malignant progression of muscle-invasive transitional cell carcinoma by regulating mTOR/DEPTOR pathway
- PMID: 26742010
- PMCID: PMC4742580
- DOI: 10.1038/bjc.2015.444
Regulator of cullins-1 expression knockdown suppresses the malignant progression of muscle-invasive transitional cell carcinoma by regulating mTOR/DEPTOR pathway
Abstract
Background: Regulator of cullins-1 (ROC1) is a key subunit in the cullin-RING ligase (CRL) protein complex. Our previous study indicated that ROC1 was essential for bladder cancer cell survival and that ROC1 knockdown inhibited CRL activity, triggering G2 phase arrest and senescence. However, the role of ROC1 in the malignant progression of bladder cancer remained unknown.
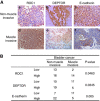
Methods: ROC1 expression in cancer cells was knocked down by siRNA silencing. The effects of ROC1 silencing were evaluated by in vitro assays for cell migration and by an in vivo mouse metastasis model. Epithelial-mesenchymal transition (EMT) induction was evaluated by immunofluorescence staining and western blotting of EMT-associated proteins. ROC1 expression in human tumours was further evaluated by immunohistochemical analysis.
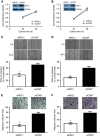
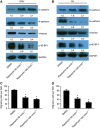
Results: ROC1 knockdown suppresses bladder cancer cell migration by inhibiting EMT. ROC1 knockdown inhibited EMT by inhibiting mammalian target of rapamycin (mTOR) activity via the accumulation of the mTOR-inhibitory protein DEPTOR, a CRL substrate. DEPTOR knockdown partially rescued ROC1 knockdown-inhibited EMT and the ROC1-induced inhibition of cancer cell migration. Furthermore, in vivo studies using a nude mouse metastasis model confirmed the in vitro data. Finally, tissue microarray analysis of clinical bladder cancer specimens indicated a positive correlation between ROC1 expression and EMT.
Conclusions: ROC1 has an important role in the malignant progression of bladder cancer via the mTOR/DEPTOR pathway. ROC1 may serve as a novel therapeutic target for the treatment of muscle-invasive transitional cell carcinoma.
Figures



References
-
- Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M, Wang XA, Zhang F, Jiang L, Zhang Y, Hu Y, Xiang S, Shu Y, Bao R, Li H, Wu W, Weng H, Yen Y, Liu Y (2015) Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett 360(2): 141–150. - PubMed
-
- Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, Lyssiotis CA, Gygi SP, Toker A, Cantley LC, Asara JM, Harper JW, Wei W (2011) mTOR drives its own activation via SCF(betaTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell 44(2): 290–303. - PMC - PubMed
-
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12(2): 89–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

