Lesinurad in combination with allopurinol: results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol
- PMID: 26742777
- PMCID: PMC4893096
- DOI: 10.1136/annrheumdis-2015-207919
Lesinurad in combination with allopurinol: results of a phase 2, randomised, double-blind study in patients with gout with an inadequate response to allopurinol
Abstract
Objectives: To assess the efficacy and tolerability of lesinurad, an oral selective uric acid reabsorption inhibitor, in combination with allopurinol versus allopurinol alone in patients with gout and an inadequate response to allopurinol.
Methods: Patients (N=227) with an inadequate response to allopurinol, defined as serum urate (sUA) ≥6 mg/dL on ≥2 occasions ≥2 weeks apart despite ≥6 weeks of allopurinol, were randomised 2:1 to 4 weeks of double-blind treatment with lesinurad (200, 400 or 600 mg/day) or matching placebo in combination with their prestudy allopurinol dose (200-600 mg/day). Colchicine prophylaxis for gout flares was required. The primary end point was percent reduction from baseline sUA levels at 4 weeks. A pharmacokinetic substudy was also conducted. Safety was assessed throughout.
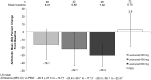
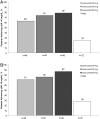
Results: Patients (n=208) received ≥1 dose of blinded medication. Lesinurad 200, 400 and 600 mg in combination with allopurinol produced significant mean percent reductions from baseline sUA of 16%, 22% and 30%, respectively, versus a mean 3% increase with placebo (p<0.0001, all doses vs placebo). Similar results were observed in patients with mild or moderate renal insufficiency (estimated creatinine clearance 30 to <90 mL/min). The incidence of ≥1 treatment-emergent adverse event was 46%, 48% and 54% with lesinurad 200, 400 and 600 mg, respectively, and 46% with placebo (most frequent, gout flares, arthralgia, headache and nasopharyngitis), with no deaths or serious adverse events.
Conclusions: Lesinurad achieves clinically relevant and statistically significant reductions in sUA in combination with allopurinol in patients who warrant additional therapy on allopurinol alone.
Trial registration number: NCT01001338.
Keywords: Gout; Inflammation; Pharmacokinetics; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

