Protein-targeted corona phase molecular recognition
- PMID: 26742890
- PMCID: PMC4729864
- DOI: 10.1038/ncomms10241
Protein-targeted corona phase molecular recognition
Abstract
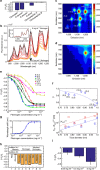
Corona phase molecular recognition (CoPhMoRe) uses a heteropolymer adsorbed onto and templated by a nanoparticle surface to recognize a specific target analyte. This method has not yet been extended to macromolecular analytes, including proteins. Herein we develop a variant of a CoPhMoRe screening procedure of single-walled carbon nanotubes (SWCNT) and use it against a panel of human blood proteins, revealing a specific corona phase that recognizes fibrinogen with high selectivity. In response to fibrinogen binding, SWCNT fluorescence decreases by >80% at saturation. Sequential binding of the three fibrinogen nodules is suggested by selective fluorescence quenching by isolated sub-domains and validated by the quenching kinetics. The fibrinogen recognition also occurs in serum environment, at the clinically relevant fibrinogen concentrations in the human blood. These results open new avenues for synthetic, non-biological antibody analogues that recognize biological macromolecules, and hold great promise for medical and clinical applications.
Figures



References
-
- de Silva A. P. et al. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997) . - PubMed
-
- Lavigne J. J. & Anslyn E. V. Sensing a paradigm shift in the field of molecular recognition: from selective to differential receptors. Angew. Chem. Int. Ed. 40, 3118–3130 (2001) . - PubMed
-
- Alexander C., Davidson L. & Hayes W. Imprinted polymers: artificial molecular recognition materials with applications in synthesis and catalysis. Tetrahedron 59, 2025–2057 (2003) .
-
- Kumar A., Sun S.-S. & Lees A. J. Directed assembly metallocyclic supramolecular systems for molecular recognition and chemical sensing. Coord. Chem. Rev. 252, 922–939 (2008) .
-
- Grzelczak M., Vermant J., Furst E. M. & Liz-Marzán L. M. Directed self-assembly of nanoparticles. ACS Nano 4, 3591–3605 (2010) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

