Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study
- PMID: 26743120
- PMCID: PMC4740728
- DOI: 10.1530/ERC-15-0490
Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study
Abstract
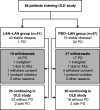
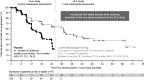
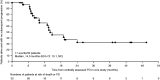
In the CLARINET study, lanreotide Autogel (depot in USA) significantly prolonged progression-free survival (PFS) in patients with metastatic pancreatic/intestinal neuroendocrine tumours (NETs). We report long-term safety and additional efficacy data from the open-label extension (OLE). Patients with metastatic grade 1/2 (Ki-67 ≤ 10%) non-functioning NET and documented baseline tumour-progression status received lanreotide Autogel 120 mg (n = 101) or placebo (n = 103) for 96 weeks or until death/progressive disease (PD) in CLARINET study. Patients with stable disease (SD) at core study end (lanreotide/placebo) or PD (placebo only) continued or switched to lanreotide in the OLE. In total, 88 patients (previously: lanreotide, n = 41; placebo, n = 47) participated: 38% had pancreatic, 39% midgut and 23% other/unknown primary tumours. Patients continuing lanreotide reported fewer adverse events (AEs) (all and treatment-related) during OLE than core study. Placebo-to-lanreotide switch patients reported similar AE rates in OLE and core studies, except more diarrhoea was considered treatment-related in OLE (overall diarrhoea unchanged). Median lanreotide PFS (core study randomisation to PD in core/OLE; n=101) was 32.8 months (95% CI: 30.9, 68.0). A sensitivity analysis, addressing potential selection bias by assuming that patients with SD on lanreotide in the core study and not entering the OLE (n=13) had PD 24 weeks after last core assessment, found median PFS remaining consistent: 30.8 months (95% CI: 30.0, 31.3). Median time to further PD after placebo-to-lanreotide switch (n=32) was 14.0 months (10.1; not reached). This OLE study suggests long-term treatment with lanreotide Autogel 120 mg maintained favourable safety/tolerability. CLARINET OLE data also provide new evidence of lanreotide anti-tumour benefits in indolent and progressive pancreatic/intestinal NETs.
Keywords: anti-tumour effects; lanreotide Autogel; neuroendocrine tumours; open-label extension.
© 2016 The authors.
Figures
References
-
- Bajetta E, Procopio G, Catena L, Martinetti A, De DS, Ricci S, Lecchi AS, Boscani PF, Iacobelli S, Carteni G, et al. Lanreotide autogel every 6 weeks compared with Lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: a Phase III Study. Cancer. 2006;107:2474–2481. doi: 10.1002/cncr.22272. - DOI - PubMed
-
- Food and Drug Administration (FDA) 2007 Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. Rockville, MD, USA: U.S. Department of Health and Human Services, Food and Drug Administration. (available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati...)
-
- Ipsen Biopharmaceuticals, Inc. 2014 SOMATULINE DEPOT labeling revision 12/22/2014 Reference ID: 3677425. Basking Ridge, NJ, USA: Ipsen Biopharmaceuticals, Inc. (available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s010lbl.pdf)
-
- Ipsen Ltd. 2015 Somatuline® Autogel®. Summary of product characteristics (SmPC). Slough, Berkshire, UK: Ipsen Ltd. (available at: http://www.medicines.org.uk/emc/medicine/25104)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

