MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides
- PMID: 26744218
- PMCID: PMC4790871
- DOI: 10.1105/tpc.15.00706
MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides
Abstract
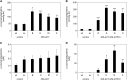
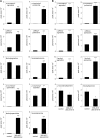
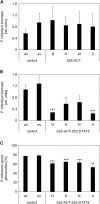
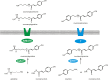
The ability of Arabidopsis thaliana to successfully prevent colonization by Phytophthora infestans, the causal agent of late blight disease of potato (Solanum tuberosum), depends on multilayered defense responses. To address the role of surface-localized secondary metabolites for entry control, droplets of a P. infestans zoospore suspension, incubated on Arabidopsis leaves, were subjected to untargeted metabolite profiling. The hydroxycinnamic acid amide coumaroylagmatine was among the metabolites secreted into the inoculum. In vitro assays revealed an inhibitory activity of coumaroylagmatine on P. infestans spore germination. Mutant analyses suggested a requirement of the p-coumaroyl-CoA:agmatine N4-p-coumaroyl transferase ACT for the biosynthesis and of the MATE transporter DTX18 for the extracellular accumulation of coumaroylagmatine. The host plant potato is not able to efficiently secrete coumaroylagmatine. This inability is overcome in transgenic potato plants expressing the two Arabidopsis genes ACT and DTX18. These plants secrete agmatine and putrescine conjugates to high levels, indicating that DTX18 is a hydroxycinnamic acid amide transporter with a distinct specificity. The export of hydroxycinnamic acid amides correlates with a decreased ability of P. infestans spores to germinate, suggesting a contribution of secreted antimicrobial compounds to pathogen defense at the leaf surface.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures






Similar articles
-
The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity.Front Plant Sci. 2022 Jun 22;13:922119. doi: 10.3389/fpls.2022.922119. eCollection 2022. Front Plant Sci. 2022. PMID: 35812905 Free PMC article. Review.
-
Albugo-imposed changes to tryptophan-derived antimicrobial metabolite biosynthesis may contribute to suppression of non-host resistance to Phytophthora infestans in Arabidopsis thaliana.BMC Biol. 2017 Mar 20;15(1):20. doi: 10.1186/s12915-017-0360-z. BMC Biol. 2017. PMID: 28320402 Free PMC article.
-
Quantitative resistance in potato leaves to late blight associated with induced hydroxycinnamic acid amides.Funct Integr Genomics. 2014 Jun;14(2):285-98. doi: 10.1007/s10142-013-0358-8. Epub 2014 Jan 10. Funct Integr Genomics. 2014. PMID: 24408130
-
Early Pep-13-induced immune responses are SERK3A/B-dependent in potato.Sci Rep. 2019 Dec 5;9(1):18380. doi: 10.1038/s41598-019-54944-y. Sci Rep. 2019. PMID: 31804581 Free PMC article.
-
p-Coumaroyl Amides from the Plant Kingdom: A Comprehensive Review of Natural Sources, Biosynthesis, and Biological Activities.Molecules. 2025 Mar 11;30(6):1259. doi: 10.3390/molecules30061259. Molecules. 2025. PMID: 40142036 Free PMC article. Review.
Cited by
-
The suberin transporter StABCG1 is required for barrier formation in potato leaves.Sci Rep. 2025 Mar 7;15(1):7930. doi: 10.1038/s41598-025-89032-x. Sci Rep. 2025. PMID: 40050620 Free PMC article.
-
The Arabidopsis non-host defence-associated coumarin scopoletin protects soybean from Asian soybean rust.Plant J. 2019 Aug;99(3):397-413. doi: 10.1111/tpj.14426. Epub 2019 Jul 1. Plant J. 2019. PMID: 31148306 Free PMC article.
-
The Pathogen-Induced MATE Gene TaPIMA1 Is Required for Defense Responses to Rhizoctonia cerealis in Wheat.Int J Mol Sci. 2022 Mar 21;23(6):3377. doi: 10.3390/ijms23063377. Int J Mol Sci. 2022. PMID: 35328796 Free PMC article.
-
The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity.Front Plant Sci. 2022 Jun 22;13:922119. doi: 10.3389/fpls.2022.922119. eCollection 2022. Front Plant Sci. 2022. PMID: 35812905 Free PMC article. Review.
-
Metabolo-transcriptome profiling of barley reveals induction of chitin elicitor receptor kinase gene (HvCERK1) conferring resistance against Fusarium graminearum.Plant Mol Biol. 2017 Feb;93(3):247-267. doi: 10.1007/s11103-016-0559-3. Epub 2016 Nov 14. Plant Mol Biol. 2017. PMID: 27844244
References
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Bednarek P. (2012). Sulfur-containing secondary metabolites from Arabidopsis thaliana and other Brassicaceae with function in plant immunity. ChemBioChem 13: 1846–1859. - PubMed
-
- Böttcher C., von Roepenack-Lahaye E., Schmidt J., Schmotz C., Neumann S., Scheel D., Clemens S. (2008). Metabolome analysis of biosynthetic mutants reveals a diversity of metabolic changes and allows identification of a large number of new compounds in Arabidopsis. Plant Physiol. 147: 2107–2120. - PMC - PubMed
-
- Böttcher C., Westphal L., Schmotz C., Prade E., Scheel D., Glawischnig E. (2009). The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 21: 1830–1845. - PMC - PubMed
-
- Burhenne K., Kristensen B.K., Rasmussen S.K. (2003). A new class of N-hydroxycinnamoyltransferases. Purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3.1.64). J. Biol. Chem. 278: 13919–13927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

