Mechanistic Investigations into the Application of Sulfoxides in Carbohydrate Synthesis
- PMID: 26744250
- PMCID: PMC4794778
- DOI: 10.1002/chem.201503504
Mechanistic Investigations into the Application of Sulfoxides in Carbohydrate Synthesis
Abstract
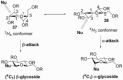
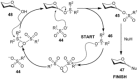
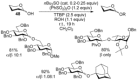
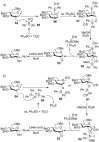
The utility of sulfoxides in a diverse range of transformations in the field of carbohydrate chemistry has seen rapid growth since the first introduction of a sulfoxide as a glycosyl donor in 1989. Sulfoxides have since developed into more than just anomeric leaving groups, and today have multiple roles in glycosylation reactions. These include as activators for thioglycosides, hemiacetals, and glycals, and as precursors to glycosyl triflates, which are essential for stereoselective β-mannoside synthesis, and bicyclic sulfonium ions that facilitate the stereoselective synthesis of α-glycosides. In this review we highlight the mechanistic investigations undertaken in this area, often outlining strategies employed to differentiate between multiple proposed reaction pathways, and how the conclusions of these investigations have and continue to inform upon the development of more efficient transformations in sulfoxide-based carbohydrate synthesis.
Keywords: carbohydrates; glycosylation; mechanism; organic synthesis; sulfoxides.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
















Similar articles
-
Mechanistic studies on a sulfoxide transfer reaction mediated by diphenyl sulfoxide/triflic anhydride.Chemistry. 2012 Mar 5;18(10):2987-97. doi: 10.1002/chem.201102861. Epub 2012 Jan 31. Chemistry. 2012. PMID: 22294491 Free PMC article.
-
Glycosylation from the non-reducing end using a combination of thioglycoside and glycosyl sulfoxide as the glycosyl donor and the acceptor.Chem Pharm Bull (Tokyo). 2010 May;58(5):758-64. doi: 10.1248/cpb.58.758. Chem Pharm Bull (Tokyo). 2010. PMID: 20460812
-
AuCl3-AgOTf promoted O-glycosylation using anomeric sulfoxides as glycosyl donors at room temperature.Carbohydr Res. 2017 Jan 2;437:43-49. doi: 10.1016/j.carres.2016.11.012. Epub 2016 Nov 23. Carbohydr Res. 2017. PMID: 27912141
-
Synthetic carbohydrate research based on organic electrochemistry.Carbohydr Res. 2012 Dec 1;363:1-6. doi: 10.1016/j.carres.2012.09.023. Epub 2012 Oct 6. Carbohydr Res. 2012. PMID: 23089173 Review.
-
Aromatic O-glycosylation.Carbohydr Res. 2006 Jul 24;341(10):1266-81. doi: 10.1016/j.carres.2006.04.004. Epub 2006 May 2. Carbohydr Res. 2006. PMID: 16650391 Review.
Cited by
-
Mapping the Reactivity and Selectivity of 2-Azidofucosyl Donors for the Assembly of N-Acetylfucosamine-Containing Bacterial Oligosaccharides.J Org Chem. 2017 Jan 20;82(2):848-868. doi: 10.1021/acs.joc.6b02593. Epub 2017 Jan 10. J Org Chem. 2017. PMID: 28051314 Free PMC article.
-
Why Are 5-Thioglycopyranosyl Donors More Axially Selective than their Glycopyranosyl Counterparts? A Low and Variable Temperature NMR Spectroscopy and Computational Study.JACS Au. 2025 Jan 23;5(2):871-889. doi: 10.1021/jacsau.4c01113. eCollection 2025 Feb 24. JACS Au. 2025. PMID: 40017772 Free PMC article.
-
Practical Glucosylations and Mannosylations Using Anomeric Benzoyloxy as a Leaving Group Activated by Sulfonium Ion.ACS Omega. 2017 Jul 31;2(7):3698-3709. doi: 10.1021/acsomega.7b00729. Epub 2017 Jul 18. ACS Omega. 2017. PMID: 30023701 Free PMC article.
-
The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1-SN2 Interface.Chem Rev. 2018 Sep 12;118(17):8242-8284. doi: 10.1021/acs.chemrev.8b00083. Epub 2018 May 30. Chem Rev. 2018. PMID: 29846062 Free PMC article. Review.
-
Synthesis of a Pseudodisaccharide Suitable for Synthesis of Ring I Modified 4,5-2-Deoxystreptamine Type Aminoglycoside Antibiotics.J Org Chem. 2020 Jun 5;85(11):7583-7587. doi: 10.1021/acs.joc.0c00743. Epub 2020 May 8. J Org Chem. 2020. PMID: 32336094 Free PMC article.
References
-
- None
-
- Tsuchihashi G., Int. J. Sulfur Chem. Part B 1972, 7, 185–186;
-
- Griffiths S. L., Marcos C. F., Perrio S., Saberi S. P., Thomas S. E., Tustin G. J., Wierzchleyski A. T., Pure Appl. Chem. 1994, 66, 1565–1572;
-
- Carreno M. C., Chem. Rev. 1995, 95, 1717–1760.
-
- Tidwell T. T., Synthesis 1990, 857–870.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

