LRRK2 BAC transgenic rats develop progressive, L-DOPA-responsive motor impairment, and deficits in dopamine circuit function
- PMID: 26744332
- PMCID: PMC4754049
- DOI: 10.1093/hmg/ddv628
LRRK2 BAC transgenic rats develop progressive, L-DOPA-responsive motor impairment, and deficits in dopamine circuit function
Abstract
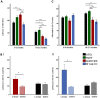
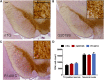
Mutations in leucine-rich repeat kinase 2 (LRRK2) lead to late-onset, autosomal dominant Parkinson's disease, characterized by the degeneration of dopamine neurons of the substantia nigra pars compacta, a deficit in dopamine neurotransmission and the development of motor and non-motor symptoms. The most prevalent Parkinson's disease LRRK2 mutations are located in the kinase (G2019S) and GTPase (R1441C) encoding domains of LRRK2. To better understand the sequence of events that lead to progressive neurophysiological deficits in vulnerable neurons and circuits in Parkinson's disease, we have generated LRRK2 bacterial artificial chromosome transgenic rats expressing either G2019S or R1441C mutant, or wild-type LRRK2, from the complete human LRRK2 genomic locus, including endogenous promoter and regulatory regions. Aged (18-21 months) G2019S and R1441C mutant transgenic rats exhibit L-DOPA-responsive motor dysfunction, impaired striatal dopamine release as determined by fast-scan cyclic voltammetry, and cognitive deficits. In addition, in vivo recordings of identified substantia nigra pars compacta dopamine neurons in R1441C LRRK2 transgenic rats reveal an age-dependent reduction in burst firing, which likely results in further reductions to striatal dopamine release. These alterations to dopamine circuit function occur in the absence of neurodegeneration or abnormal protein accumulation within the substantia nigra pars compacta, suggesting that nigrostriatal dopamine dysfunction precedes detectable protein aggregation and cell death in the development of Parkinson's disease. In conclusion, our longitudinal deep-phenotyping provides novel insights into how the genetic burden arising from human mutant LRRK2 manifests as early pathophysiological changes to dopamine circuit function and highlights a potential model for testing Parkinson's therapeutics.
© The Author 2016. Published by Oxford University Press.
Figures



References
-
- Haugarvoll K., Wszolek Z.K. (2009) Clinical features of LRRK2 parkinsonism. Parkinsonism Relat. Disord., 15(Suppl. 3), S205–S208. - PubMed
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B. et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 44, 601–607. - PubMed
-
- Gilks W.P., Abou-Sleiman P.M., Gandhi S., Jain S., Singleton A., Lees A.J., Shaw K., Bhatia K.P., Bonifati V., Quinn N.P. et al. (2005) A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet, 365, 415–416. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- MC_U138197109/MRC_/Medical Research Council/United Kingdom
- G0700932/MRC_/Medical Research Council/United Kingdom
- G-1003/PUK_/Parkinson's UK/United Kingdom
- H-1003/PUK_/Parkinson's UK/United Kingdom
- MC_U138164490/MRC_/Medical Research Council/United Kingdom
- MC_UU_12020/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12020/5/MRC_/Medical Research Council/United Kingdom
- G-1103/PUK_/Parkinson's UK/United Kingdom
- G-1504/PUK_/Parkinson's UK/United Kingdom
- MC_UU_12024/2/MRC_/Medical Research Council/United Kingdom
- MR/J004324/1/MRC_/Medical Research Council/United Kingdom
- J-0901/PUK_/Parkinson's UK/United Kingdom
- G-0803/PUK_/Parkinson's UK/United Kingdom
- U138164490/MRC_/Medical Research Council/United Kingdom
- U138197109/MRC_/Medical Research Council/United Kingdom
- G-1305/PUK_/Parkinson's UK/United Kingdom
- G-0808/PUK_/Parkinson's UK/United Kingdom
- MR/K013866/1/MRC_/Medical Research Council/United Kingdom
- 087736/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

