Mitochondria-associated membranes as hubs for neurodegeneration
- PMID: 26744348
- PMCID: PMC4789254
- DOI: 10.1007/s00401-015-1528-7
Mitochondria-associated membranes as hubs for neurodegeneration
Abstract
There is a growing appreciation that membrane-bound organelles in eukaryotic cells communicate directly with one another through direct membrane contact sites. Mitochondria-associated membranes are specialized subdomains of the endoplasmic reticulum that function as membrane contact sites between the endoplasmic reticulum and mitochondria. These sites have emerged as major players in lipid metabolism and calcium signaling. More recently also autophagy and mitochondrial dynamics have been found to be regulated at ER-mitochondria contact sites. Neurons critically depend on mitochondria-associated membranes as a means to exchange metabolites and signaling molecules between these organelles. This is underscored by the fact that genes affecting mitochondrial and endoplasmic reticulum homeostasis are clearly overrepresented in several hereditary neurodegenerative disorders. Conversely, the processes affected by the contact sites between the endoplasmic reticulum and mitochondria are widely implicated in neurodegeneration. This review will focus on the most recent data addressing the structural composition and function of the mitochondria-associated membranes. In addition, the 3D morphology of the contact sites as observed using volume electron microscopy is discussed. Finally, it will highlight the role of several key proteins associated with these contact sites that are involved not only in dementias, amyotrophic lateral sclerosis and Parkinson's disease, but also in axonopathies such as hereditary spastic paraplegia and Charcot-Marie-Tooth disease.
Figures
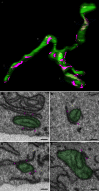
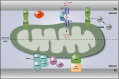
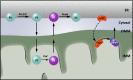



References
-
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem FEBS. 1999;264:545–553. doi: 10.1046/j.1432-1327.1999.00658.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

