Host-pathogen interactions in bacterial meningitis
- PMID: 26744349
- PMCID: PMC4713723
- DOI: 10.1007/s00401-015-1531-z
Host-pathogen interactions in bacterial meningitis
Abstract
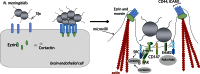
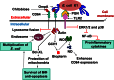
Bacterial meningitis is a devastating disease occurring worldwide with up to half of the survivors left with permanent neurological sequelae. Due to intrinsic properties of the meningeal pathogens and the host responses they induce, infection can cause relatively specific lesions and clinical syndromes that result from interference with the function of the affected nervous system tissue. Pathogenesis is based on complex host-pathogen interactions, some of which are specific for certain bacteria, whereas others are shared among different pathogens. In this review, we summarize the recent progress made in understanding the molecular and cellular events involved in these interactions. We focus on selected major pathogens, Streptococcus pneumonia, S. agalactiae (Group B Streptococcus), Neisseria meningitidis, and Escherichia coli K1, and also include a neglected zoonotic pathogen, Streptococcus suis. These neuroinvasive pathogens represent common themes of host-pathogen interactions, such as colonization and invasion of mucosal barriers, survival in the blood stream, entry into the central nervous system by translocation of the blood-brain and blood-cerebrospinal fluid barrier, and induction of meningeal inflammation, affecting pia mater, the arachnoid and subarachnoid spaces.
Keywords: Bacterial meningitis; Escherichia coli K1; Group B Streptococcus; Meningococci; Neuroinfectiology; Pneumococci; Streptococcus suis.
Figures



References
-
- Baker CJ, Edwards MS. Group B streptococcal infections. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. 5. Philadelphia: WB Saunders; 2001. pp. 1091–1156.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

