Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model
- PMID: 26744352
- PMCID: PMC4770151
- DOI: 10.1242/dmm.023275
Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model
Abstract
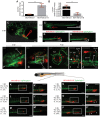
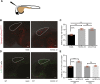
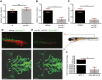
Triple-negative breast cancer (TNBC) is a highly aggressive and recurrent type of breast carcinoma that is associated with poor patient prognosis. Because of the limited efficacy of current treatments, new therapeutic strategies need to be developed. The CXCR4-CXCL12 chemokine signaling axis guides cell migration in physiological and pathological processes, including breast cancer metastasis. Although targeted therapies to inhibit the CXCR4-CXCL12 axis are under clinical experimentation, still no effective therapeutic approaches have been established to block CXCR4 in TNBC. To unravel the role of the CXCR4-CXCL12 axis in the formation of TNBC early metastases, we used the zebrafish xenograft model. Importantly, we demonstrate that cross-communication between the zebrafish and human ligands and receptors takes place and human tumor cells expressing CXCR4 initiate early metastatic events by sensing zebrafish cognate ligands at the metastatic site. Taking advantage of the conserved intercommunication between human tumor cells and the zebrafish host, we blocked TNBC early metastatic events by chemical and genetic inhibition of CXCR4 signaling. We used IT1t, a potent CXCR4 antagonist, and show for the first time its promising anti-tumor effects. In conclusion, we confirm the validity of the zebrafish as a xenotransplantation model and propose a pharmacological approach to target CXCR4 in TNBC.
Keywords: CXCL12; CXCR4; IT1t; Inter-species crosstalk; Metastases; Triple-negative breast cancer; Xenograft; Zebrafish.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



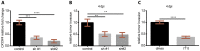
References
-
- Balabanian K., Lagane B., Infantino S., Chow K. Y. C., Harriague J., Moepps B., Arenzana-Seisdedos F., Thelen M. and Bachelerie F. (2005). The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 280, 35760-35766. 10.1074/jbc.M508234200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

