Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation
- PMID: 26744461
- PMCID: PMC4797023
- DOI: 10.1182/blood-2015-08-663146
Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation
Abstract
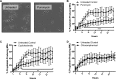
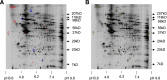
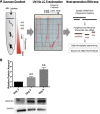
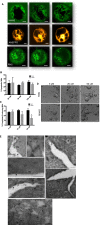
Platelets are essential for hemostasis, and thrombocytopenia is a major clinical problem. Megakaryocytes (MKs) generate platelets by extending long processes, proplatelets, into sinusoidal blood vessels. However, very little is known about what regulates proplatelet formation. To uncover which proteins were dynamically changing during this process, we compared the proteome and transcriptome of round vs proplatelet-producing MKs by 2D difference gel electrophoresis (DIGE) and polysome profiling, respectively. Our data revealed a significant increase in a poorly-characterized MK protein, myristoylated alanine-rich C-kinase substrate (MARCKS), which was upregulated 3.4- and 5.7-fold in proplatelet-producing MKs in 2D DIGE and polysome profiling analyses, respectively. MARCKS is a protein kinase C (PKC) substrate that binds PIP2. In MKs, it localized to both the plasma and demarcation membranes. MARCKS inhibition by peptide significantly decreased proplatelet formation 53%. To examine the role of MARCKS in the PKC pathway, we treated MKs with polymethacrylate (PMA), which markedly increased MARCKS phosphorylation while significantly inhibiting proplatelet formation 84%, suggesting that MARCKS phosphorylation reduces proplatelet formation. We hypothesized that MARCKS phosphorylation promotes Arp2/3 phosphorylation, which subsequently downregulates proplatelet formation; both MARCKS and Arp2 were dephosphorylated in MKs making proplatelets, and Arp2 inhibition enhanced proplatelet formation. Finally, we used MARCKS knockout (KO) mice to probe the direct role of MARCKS in proplatelet formation; MARCKS KO MKs displayed significantly decreased proplatelet levels. MARCKS expression and signaling in primary MKs is a novel finding. We propose that MARCKS acts as a "molecular switch," binding to and regulating PIP2 signaling to regulate processes like proplatelet extension (microtubule-driven) vs proplatelet branching (Arp2/3 and actin polymerization-driven).
Figures


References
-
- Machlus KR, Thon JN, Italiano JE., Jr Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. 2014;165(2):227–236. - PubMed
-
- DHHS. The 2011 national blood collection and utilization survey report. Department of Health and Human Services; 2013.
-
- Thon JN, Macleod H, Begonja AJ, et al. Microtubule and cortical forces determine platelet size during vascular platelet production. Nat Commun. 2012;3:852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

