Endothelial and Smooth Muscle Cell Interactions in the Pathobiology of Pulmonary Hypertension
- PMID: 26744837
- PMCID: PMC4821060
- DOI: 10.1165/rcmb.2015-0323TR
Endothelial and Smooth Muscle Cell Interactions in the Pathobiology of Pulmonary Hypertension
Abstract
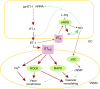
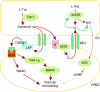
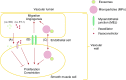
In the pulmonary vasculature, the endothelial and smooth muscle cells are two key cell types that play a major role in the pathobiology of pulmonary vascular disease and pulmonary hypertension. The normal interactions between these two cell types are important for the homeostasis of the pulmonary circulation, and any aberrant interaction between them may lead to various disease states including pulmonary vascular remodeling and pulmonary hypertension. It is well recognized that the endothelial cell can regulate the function of the underlying smooth muscle cell by releasing various bioactive agents such as nitric oxide and endothelin-1. In addition to such paracrine regulation, other mechanisms exist by which there is cross-talk between these two cell types, including communication via the myoendothelial injunctions and information transfer via extracellular vesicles. Emerging evidence suggests that these nonparacrine mechanisms play an important role in the regulation of pulmonary vascular tone and the determination of cell phenotype and that they are critically involved in the pathobiology of pulmonary hypertension.
Keywords: microvesicles; myoendothelial injunction; paracrine; vascular remodeling; vasoconstriction.
Figures

References
-
- Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90:1291–1335. - PubMed
-
- Campbell WB, Harder DR. Prologue: EDHF—what is it? Am J Physiol Heart Circ Physiol. 2001;280:H2413–H2416. - PubMed
-
- Dora KA. Cell-cell communication in the vessel wall. Vasc Med. 2001;6:43–50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

