The REST remodeling complex protects genomic integrity during embryonic neurogenesis
- PMID: 26745185
- PMCID: PMC4728133
- DOI: 10.7554/eLife.09584
The REST remodeling complex protects genomic integrity during embryonic neurogenesis
Abstract
The timely transition from neural progenitor to post-mitotic neuron requires down-regulation and loss of the neuronal transcriptional repressor, REST. Here, we have used mice containing a gene trap in the Rest gene, eliminating transcription from all coding exons, to remove REST prematurely from neural progenitors. We find that catastrophic DNA damage occurs during S-phase of the cell cycle, with long-term consequences including abnormal chromosome separation, apoptosis, and smaller brains. Persistent effects are evident by latent appearance of proneural glioblastoma in adult mice deleted additionally for the tumor suppressor p53 protein (p53). A previous line of mice deleted for REST in progenitors by conventional gene targeting does not exhibit these phenotypes, likely due to a remaining C-terminal peptide that still binds chromatin and recruits co-repressors. Our results suggest that REST-mediated chromatin remodeling is required in neural progenitors for proper S-phase dynamics, as part of its well-established role in repressing neuronal genes until terminal differentiation.
Keywords: REST complex; developmental biology; genomic instability; knockout animals; mouse; neurogenesis; repression; stem cells; transcription factors.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
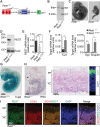

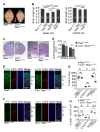

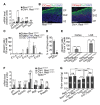
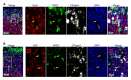

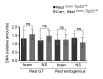
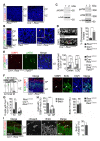
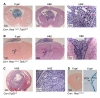


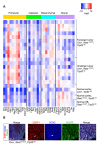
Comment in
-
Give it a REST!Elife. 2016 Jan 8;5:e12615. doi: 10.7554/eLife.12615. Elife. 2016. PMID: 26745186 Free PMC article.
References
-
- Andres ME, Burger C, Peral-Rubio MJ, Battaglioli E, Anderson ME, Grimes J, Dallman J, Ballas N, Mandel G. CoREST: a functional corepressor required for regulation of neural-specific gene expression. Proceedings of the National Academy of Sciences. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. - DOI - PMC - PubMed
-
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/S0896-6273(01)00371-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

