DNA damage response during mouse oocyte maturation
- PMID: 26745237
- PMCID: PMC5056612
- DOI: 10.1080/15384101.2015.1128592
DNA damage response during mouse oocyte maturation
Abstract
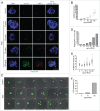
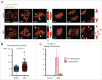
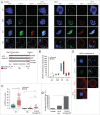
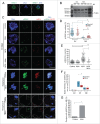
Because low levels of DNA double strand breaks (DSBs) appear not to activate the ATM-mediated prophase I checkpoint in full-grown oocytes, there may exist mechanisms to protect chromosome integrity during meiotic maturation. Using live imaging we demonstrate that low levels of DSBs induced by the radiomimetic drug Neocarzinostatin (NCS) increase the incidence of chromosome fragments and lagging chromosomes but do not lead to APC/C activation and anaphase onset delay. The number of DSBs, represented by γH2AX foci, significantly decreases between prophase I and metaphase II in both control and NCS-treated oocytes. Transient treatment with NCS increases >2-fold the number of DSBs in prophase I oocytes, but less than 30% of these oocytes enter anaphase with segregation errors. MRE11, but not ATM, is essential to detect DSBs in prophase I and is involved in H2AX phosphorylation during metaphase I. Inhibiting MRE11 by mirin during meiotic maturation results in anaphase bridges and also increases the number of γH2AX foci in metaphase II. Compromised DNA integrity in mirin-treated oocytes indicates a role for MRE11 in chromosome integrity during meiotic maturation.
Keywords: DNA damage; MRE11; double strand DNA breaks; meiotic maturation; mouse oocytes.
Figures
Similar articles
-
Different fates of oocytes with DNA double-strand breaks in vitro and in vivo.Cell Cycle. 2014;13(17):2674-80. doi: 10.4161/15384101.2015.945375. Cell Cycle. 2014. PMID: 25486355 Free PMC article.
-
Functions of the MRE11 complex in the development and maintenance of oocytes.Chromosoma. 2016 Mar;125(1):151-62. doi: 10.1007/s00412-015-0535-8. Epub 2015 Aug 1. Chromosoma. 2016. PMID: 26232174 Free PMC article.
-
Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes.Int J Mol Sci. 2020 Nov 24;21(23):8892. doi: 10.3390/ijms21238892. Int J Mol Sci. 2020. PMID: 33255251 Free PMC article.
-
The multiple roles of the Mre11 complex for meiotic recombination.Chromosome Res. 2007;15(5):551-63. doi: 10.1007/s10577-007-1147-9. Chromosome Res. 2007. PMID: 17674145 Review.
-
Activation and regulation of ATM kinase activity in response to DNA double-strand breaks.Oncogene. 2007 Dec 10;26(56):7741-8. doi: 10.1038/sj.onc.1210872. Oncogene. 2007. PMID: 18066086 Review.
Cited by
-
Distinct characteristics of the DNA damage response in mammalian oocytes.Exp Mol Med. 2024 Feb;56(2):319-328. doi: 10.1038/s12276-024-01178-2. Epub 2024 Feb 14. Exp Mol Med. 2024. PMID: 38355825 Free PMC article. Review.
-
Checkpoint Kinase 1 Is a Key Signal Transducer of DNA Damage in the Early Mammalian Cleavage Embryo.Int J Mol Sci. 2023 Apr 5;24(7):6778. doi: 10.3390/ijms24076778. Int J Mol Sci. 2023. PMID: 37047751 Free PMC article. Review.
-
Single-cell RNA sequencing of human oocytes reveals a differential transcriptomic profile associated with agar-like zona pellucida.J Ovarian Res. 2024 Jun 26;17(1):132. doi: 10.1186/s13048-024-01463-8. J Ovarian Res. 2024. PMID: 38926883 Free PMC article.
-
RanGTP and importin β regulate meiosis I spindle assembly and function in mouse oocytes.EMBO J. 2020 Jan 2;39(1):e101689. doi: 10.15252/embj.2019101689. Epub 2019 Oct 16. EMBO J. 2020. PMID: 31617608 Free PMC article.
-
Elevated sperm DNA fragmentation is correlated with an increased chromosomal aneuploidy rate of miscarried conceptus in women of advanced age undergoing fresh embryo transfer cycle.Front Endocrinol (Lausanne). 2024 Apr 8;15:1289763. doi: 10.3389/fendo.2024.1289763. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38650716 Free PMC article.
References
-
- Altmeyer M, Lukas J. To spread or not to spread-chromatin modifications in response to DNA damage. Curr Opin Genet Dev 2013; 23:156–65; PMID:23312207; http://dx.doi.org/10.1016/j.gde.2012.11.001 - DOI - PubMed
-
- Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 2011; 12:90–103; PMID:21252998; http://dx.doi.org/10.1038/nrm3047 - DOI - PMC - PubMed
-
- Rein K, Stracker TH. The MRE11 complex: an important source of stress relief. Exp Cell Res 2014; 329:162–9; PMID:25447316; http://dx.doi.org/10.1016/j.yexcr.2014.10.010 - DOI - PubMed
-
- Carson CT, Schwartz RA, Stracker TH, Lilley CE, Lee DV, Weitzman MD. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J 2003; 22:6610–20; PMID:14657032; http://dx.doi.org/10.1093/emboj/cdg630 - DOI - PMC - PubMed
-
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 2003; 22:5612–21; PMID:14532133; http://dx.doi.org/10.1093/emboj/cdg541 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
