Endogenous 24S-hydroxycholesterol modulates NMDAR-mediated function in hippocampal slices
- PMID: 26745248
- PMCID: PMC4808088
- DOI: 10.1152/jn.00890.2015
Endogenous 24S-hydroxycholesterol modulates NMDAR-mediated function in hippocampal slices
Abstract
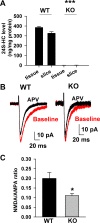
N-methyl-D-aspartate receptors (NMDARs), a major subtype of glutamate receptors mediating excitatory transmission throughout the central nervous system (CNS), play critical roles in governing brain function and cognition. Because NMDAR dysfunction contributes to the etiology of neurological and psychiatric disorders including stroke and schizophrenia, NMDAR modulators are potential drug candidates. Our group recently demonstrated that the major brain cholesterol metabolite, 24S-hydroxycholesterol (24S-HC), positively modulates NMDARs when exogenously administered. Here, we studied whether endogenous 24S-HC regulates NMDAR activity in hippocampal slices. In CYP46A1(-/-) (knockout; KO) slices where endogenous 24S-HC is greatly reduced, NMDAR tone, measured as NMDAR-to-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) excitatory postsynaptic current (EPSC) ratio, was reduced. This difference translated into more NMDAR-driven spiking in wild-type (WT) slices compared with KO slices. Application of SGE-301, a 24S-HC analog, had comparable potentiating effects on NMDAR EPSCs in both WT and KO slices, suggesting that endogenous 24S-HC does not saturate its NMDAR modulatory site in ex vivo slices. KO slices did not differ from WT slices in either spontaneous neurotransmission or in neuronal intrinsic excitability, and exhibited LTP indistinguishable from WT slices. However, KO slices exhibited higher resistance to persistent NMDAR-dependent depression of synaptic transmission induced by oxygen-glucose deprivation (OGD), an effect restored by SGE-301. Together, our results suggest that loss of positive NMDAR tone does not elicit compensatory changes in excitability or transmission, but it protects transmission against NMDAR-mediated dysfunction. We expect that manipulating this endogenous NMDAR modulator may offer new treatment strategies for neuropsychiatric dysfunction.
Keywords: 24S-hydroxycholesterol; CYP46A1 knockout mice; NMDAR; hippocampal slice.
Copyright © 2016 the American Physiological Society.
Figures






Similar articles
-
24S-hydroxycholesterol and 25-hydroxycholesterol differentially impact hippocampal neuronal survival following oxygen-glucose deprivation.PLoS One. 2017 Mar 27;12(3):e0174416. doi: 10.1371/journal.pone.0174416. eCollection 2017. PLoS One. 2017. PMID: 28346482 Free PMC article.
-
Role of Cholesterol Metabolic Enzyme CYP46A1 and Its Metabolite 24S-Hydroxycholesterol in Ischemic Stroke.Stroke. 2024 Oct;55(10):2492-2501. doi: 10.1161/STROKEAHA.124.047803. Epub 2024 Sep 3. Stroke. 2024. PMID: 39224978
-
The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors.J Neurosci. 2013 Oct 30;33(44):17290-300. doi: 10.1523/JNEUROSCI.2619-13.2013. J Neurosci. 2013. PMID: 24174662 Free PMC article.
-
24(S)-Hydroxycholesterol as a Modulator of Neuronal Signaling and Survival.Neuroscientist. 2016 Apr;22(2):132-44. doi: 10.1177/1073858414568122. Epub 2015 Jan 27. Neuroscientist. 2016. PMID: 25628343 Free PMC article. Review.
-
24S-hydroxycholesterol and cholesterol-24S-hydroxylase (CYP46A1) in the retina: from cholesterol homeostasis to pathophysiology of glaucoma.Chem Phys Lipids. 2011 Sep;164(6):496-9. doi: 10.1016/j.chemphyslip.2011.04.006. Epub 2011 Apr 21. Chem Phys Lipids. 2011. PMID: 21531213 Review.
Cited by
-
Cholesterol and oxysterols in retinal neuron-glia interactions: relevance for glaucoma.Front Ophthalmol (Lausanne). 2024 Jan 3;3:1303649. doi: 10.3389/fopht.2023.1303649. eCollection 2023. Front Ophthalmol (Lausanne). 2024. PMID: 38983043 Free PMC article. Review.
-
Regulation of synaptic function and lipid metabolism.Neural Regen Res. 2026 Mar 1;21(3):1037-1057. doi: 10.4103/NRR.NRR-D-24-01412. Epub 2025 Apr 29. Neural Regen Res. 2026. PMID: 40313084 Free PMC article.
-
Neurosteroids and oxysterols as potential therapeutic agents for glaucoma and Alzheimer's disease.Neuropsychiatry (London). 2018;8(1):344-359. doi: 10.4172/Neuropsychiatry.1000356. Neuropsychiatry (London). 2018. PMID: 30774720 Free PMC article.
-
In vitro cytochrome P450 46A1 (CYP46A1) activation by neuroactive compounds.J Biol Chem. 2017 Aug 4;292(31):12934-12946. doi: 10.1074/jbc.M117.794909. Epub 2017 Jun 22. J Biol Chem. 2017. PMID: 28642370 Free PMC article.
-
Effects of CYP46A1 Inhibition on Long-Term-Depression in Hippocampal Slices ex vivo and 24S-Hydroxycholesterol Levels in Mice in vivo.Front Mol Neurosci. 2020 Oct 29;13:568641. doi: 10.3389/fnmol.2020.568641. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33192294 Free PMC article.
References
-
- Benarroch EE. Neurosteroids: endogenous modulators of neuronal excitability and plasticity. Neurology 68: 945–947, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

