Cholesterol-Enriched Domain Formation Induced by Viral-Encoded, Membrane-Active Amphipathic Peptide
- PMID: 26745420
- PMCID: PMC4806215
- DOI: 10.1016/j.bpj.2015.11.032
Cholesterol-Enriched Domain Formation Induced by Viral-Encoded, Membrane-Active Amphipathic Peptide
Abstract
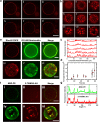
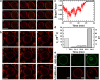
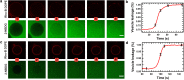
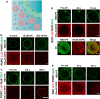
The α-helical (AH) domain of the hepatitis C virus nonstructural protein NS5A, anchored at the cytoplasmic leaflet of the endoplasmic reticulum, plays a role in viral replication. However, the peptides derived from this domain also exhibit remarkably broad-spectrum virocidal activity, raising questions about their modes of membrane association. Here, using giant lipid vesicles, we show that the AH peptide discriminates between membrane compositions. In cholesterol-containing membranes, peptide binding induces microdomain formation. By contrast, cholesterol-depleted membranes undergo global softening at elevated peptide concentrations. Furthermore, in mixed populations, the presence of ∼100 nm vesicles of viral dimensions suppresses these peptide-induced perturbations in giant unilamellar vesicles, suggesting size-dependent membrane association. These synergistic composition- and size-dependent interactions explain, in part, how the AH domain might on the one hand segregate molecules needed for viral assembly and on the other hand furnish peptides that exhibit broad-spectrum virocidal activity.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Chernomordik L.V., Vogel S.S., Zimmerberg J. Lysolipids reversibly inhibit Ca(2+)-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

